1. Duplicated muscularis mucosae (note that the latter word is “mucosae” rather than “mucosa”—this term is commonly misspelled and mispronounced) in endoscopic mucosal resection (EMR)
2. Distinguishing reactive changes from dysplastic ones
3. Identifying “true” intestinal metaplasia on hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS)/Alcian blue (AB) stains
4. Multilayered epithelium
5. Familiarity with the microscopic anatomy of the gastroesophageal (GE) junction
metaplasia. The cells in “multilayered epithelium” (19), which is discussed further below, and which may be a precursor to intestinal metaplasia, are also Alcian blue reactive.
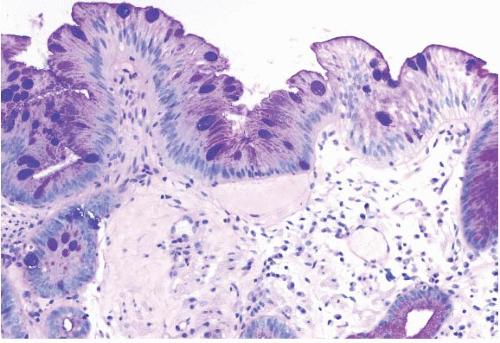
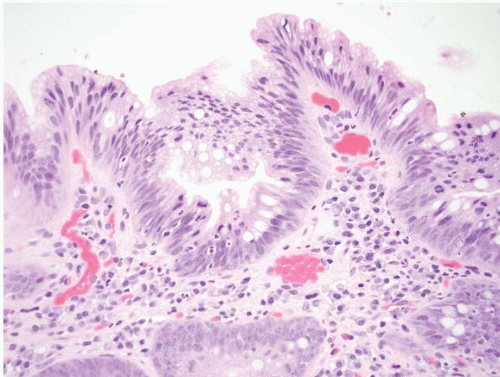
 FIGURE 1.3 Incomplete intestinal metaplasia. The metaplastic epithelium has features of both gastric and intestinal epithelium, namely goblet cells with interposed cells having foveolar-type mucin. |
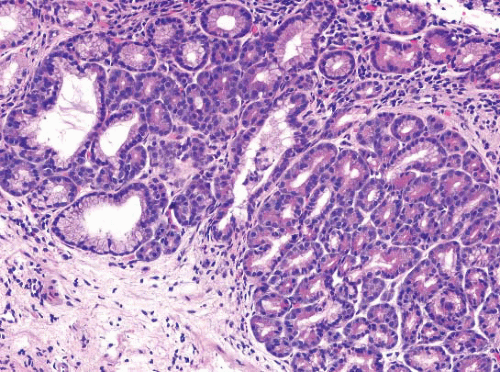
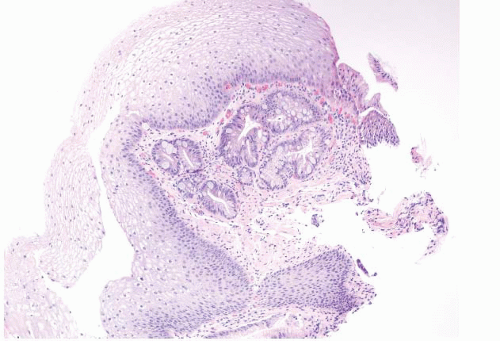
metaplasia/heterotopia in GE junction biopsies (e-Fig. 1.10) as well as oncocytic change of the submucosal glands (e-Figs. 1.11 and 1.12). Anecdotally, these changes seem to be of no clinical significance.
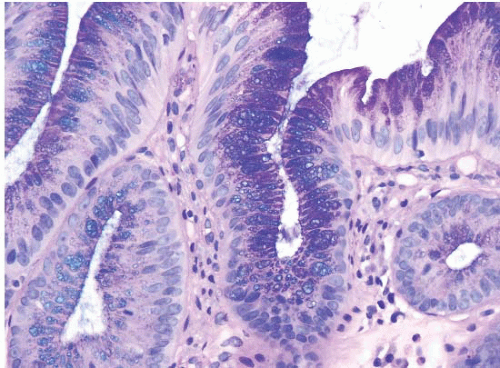
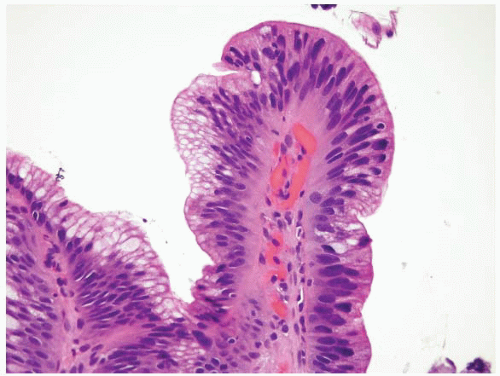
 FIGURE 1.5 Foveolar epithelium. Note the foveolar mucin cap. Some cells appear larger than others and may provoke concern for the presence of intestinal metaplasia (see Fig. 1.6). |
a. Can be recognized at endoscopy
b. Is confirmed to have intestinal metaplasia by biopsy
a. Gastric-type mucosa with no goblet cells: If it is cardia, the chances are overwhelming that it has inflammation. Most mild carditis has no known cause, although some observers believe the etiology is reflux, but some cases are attributable to Helicobacter pylori. In the absence of organisms, the appropriate diagnosis is “carditis of unknown etiology.” If the mucosa is oxyntic, it may be derived from a hiatal hernia.
b. Goblet cell containing mucosa from endoscopic tongues (e-Figs. 1.13-1.15) that the endoscopist believes is Barrett esophagus: Diagnose as “Barrett mucosa,” with the appropriate dysplasia designation (none, indefinite, low grade, high grade).
c. Goblet cell containing mucosa, but there are no endoscopic tongues although there may be a prominent, endoscopic “Z-line” (the squamocolumnar junction): Diagnose as “goblet cells at the cardia.” Some studies suggest that immunohistochemistry can help assign a designation to these, but such studies are often not practical (see below).
d. Goblet cell containing mucosa, but there is endoscopic uncertainty (i.e., the endoscopist is not sure (a) if there are tongues or (b) if there is only a prominent Z-line): Diagnose as “goblet cell containing mucosa, either Barrett mucosa or goblet cells at the cardia,” with the appropriate dysplasia designation. If the endoscopist is uncertain as to whether there is Barrett mucosa, we pathologists cannot be certain.
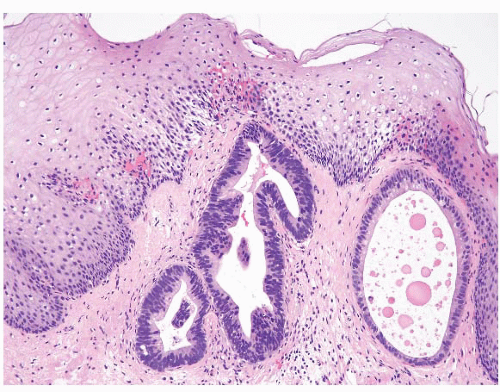
 FIGURE 1.8 Multilayered epithelium. This epithelium is characterized by four to eight layers of basally located squamous cells and associated superficial columnar, mucinous epithelium. |
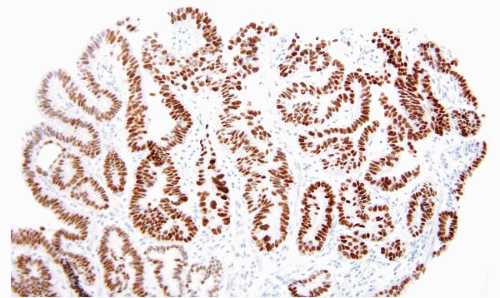
Virtually, all cases with long-segment Barrett esophagus were characterized by superficial and deep CK7 immunoreactivity (Figs. 1.10, 1.11 and 1.12, e-Figs. 1.18 and 1.19) in the intestinalized mucosa, with only superficial CK20 staining in the intestinalized zones. In contrast, distal gastric intestinal metaplasia was characterized by patchy, superficial, and deep CK20 staining in areas of incomplete intestinal metaplasia; strong, superficial, and deep CK20 staining in areas of complete intestinal metaplasia; and patchy or absent CK7 staining (e-Figs. 1.20-1.22) in either type of gastric intestinal metaplasia. As an extension of this work, this same group of
researchers evaluated the utility of CK subsets in distinguishing intestinal metaplasia of the distal esophagus from intestinal metaplasia found in the gastric cardia, in endoscopic biopsy specimens (27,28). Patients with intestinal metaplasia of the cardia all had a normal-appearing squamocolumnar junction, and biopsies of <5 mm were obtained from the esophagogastric junction, with the endoscope in a retroflexed position. The “Barrett CK7/20 pattern” (described above) was found in all cases of Barrett esophagus, but was absent in all 13 cases of cardiac intestinal metaplasia. However, other observers have questioned the utility of CK7/20 staining. The CK7 is certainly the more useful of the two, and it is critical to only stain areas with a full gland profile with intestinal metaplasia. Piazuelo et al. found CK7 staining helpful in distinguishing esophageal from gastric intestinal metaplasia (29). A study of carcinomas believed to arise in the esophagus versus the cardia, however, failed to find CK7/20 differences between these two types of cancers (30).
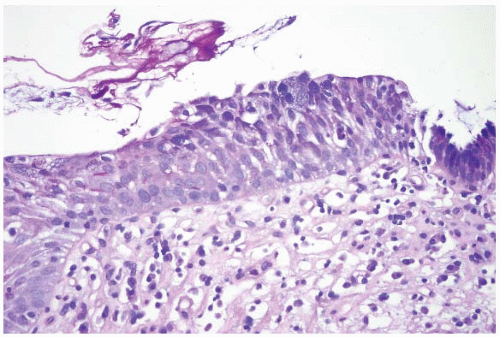 FIGURE 1.9 Multilayered epithelium (PAS/AB stain). Note superficial alcianophilic cells, corresponding to mucinous epithelium. |
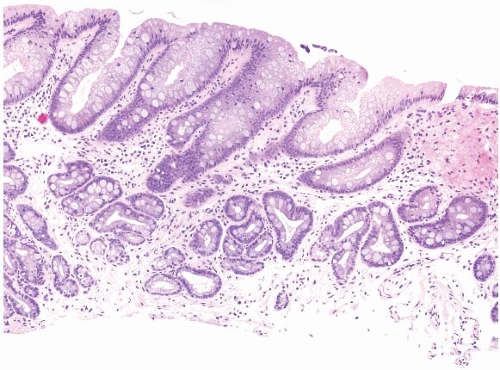 FIGURE 1.10 Biopsy from an “irregular GE junction.” It was unclear whether this tissue originated from the tubular esophagus or the GE junction. Immunohistochemical stains for CK7 and CK20 supported origin from the tubular esophagus, and thus supporting an interpretation of Barrett esophagus (see Figs. 1.11 and 1.12). This biopsy was considered negative for dysplasia due to surface maturation and abundant mucin compared with deeper portions of the crypts. There is abundant lamina propria between the glands. |
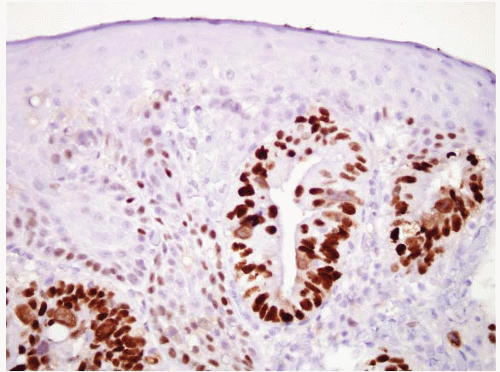
 FIGURE 1.11 CK7 staining in Barrett esophagus. Same case as Figure 1.10; note superficial and deep staining of the epithelium. |
 FIGURE 1.12 CK20 staining in Barrett esophsgus. Same case as Figures 1.10 and 1.11; note superficial CK20 staining only. |
TABLE 1.1 American College of Gastroenterology (ACG) and American Gastroenterologic Association (AGA) Guidelines for Follow-up of Barrett Esophagus | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||
1. Surface maturation compared to the underlying glands
2. Architecture of the glands
3. Cytologic features
4. Inflammation and erosions/ulcers
surface, or (d) any of the above and without certainty (a situation which would merit an interpretation of indefinite for dysplasia [IND]). Dysplastic cells are generally hyperchromatic. Once the cells have been interpreted as dysplastic, assigning a low- or high-grade category reflects a matter of degree along a morphologic continuum, a point emphasized in the 1988 criteria (36). Also included in the assessment of cytologic features is the relationship of one nucleus to another, referred to as nuclear polarity. In “normal polarity,” the long axis of the nucleus remains perpendicular to the basement membrane, and the nuclei are aligned parallel to one another. “Loss of nuclear polarity” refers to the loss of this perpendicular orientation and a random, “jumbled” appearance of the nuclei, in relation to the basement membrane and one another (Fig. 1.13).
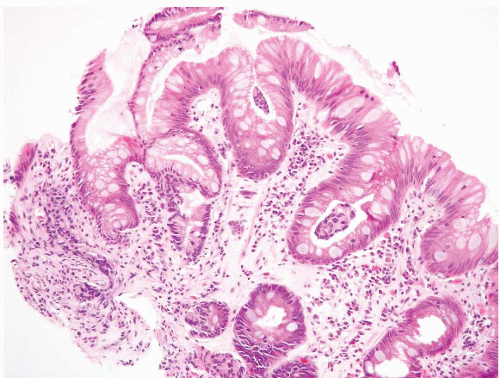
than that of the deeper glands. The architecture is normal, with abundant lamina propria between glands. The cytologic features are normal, noting that mitoses may be present in deeper glands, as well as nuclear stratification. The individual nuclei should have smooth nuclear membranes, and nucleoli, if present, should be small with smooth outlines. Nuclear polarity should be maintained in deep and superficial aspects of the biopsy. If inflammation is a component, reparative features may be present. In this setting, nuclear membranes should remain smooth, although the cells may display nuclear-to-cytoplasmic enlargement and nucleoli may become more prominent but retain smooth contours. The surface should show maturation compared to the deeper glands, but there may be some loss of surface mucin. A common pitfall in the diagnosis of Barrett esophagus is the presence of carryover intestinal mucosa from the duodenum since typically the duodenum is biopsied first with the same forceps used to biopsy the esophagus, and tissue from the intestine can contaminate the container labeled as “esophagus” (e-Figs. 1.24-1.28). These contaminants are typically small and seen detached from the esophageal mucosa. Some cases have a hypermucinous pattern reminiscent of hyperplastic polyps in the colon (e-Figs. 1.29 and 1.30).
“waste can” (36). Cases with IND could have normal architecture or some degree of glandular crowding (Figs. 1.15, e-Figs. 1.31-1.34). On cytologic evaluation, lesions could have hyperchromasia, nuclear membrane irregularities, and increased mitoses in the deeper aspects, and these matured to the surface. Loss of nuclear polarity was not a feature of IND. In the presence of inflammation, more striking architectural abnormalities were to be included in the indefinite category. This interpretation can also be applied when tangential embedding does not allow assessment between the glands and the surface. Some cases display peculiar hypermucinous features, and it is unclear whether they are neoplastic or reparative (e-Figs. 1.35 and 1.36). We often offer an explanation for resorting to this category when it is assigned.
hyperchromatic, mildly enlarged, and may show some degree of stratification and mucin loss. Mitotic figures may be seen at or close to the surface. More than mild nuclear enlargement is allowed if the other features support an interpretation of low-grade dysplasia. Loss of nuclear
polarity and nucleoli are not features of low-grade dysplasia, though nuclear stratification, similar to that seen in colonic adenomas, is within the spectrum of low-grade dysplasia and may be present at the surface (e-Figs. 1.57 and 1.58). Inflammation is typically minimal; cases with abundant inflammation and the other features of low-grade dysplasia are usually best classified in the IND category. If tangential embedding precludes evaluation of the surface, low-grade dysplasia can be diagnosed in the absence of inflammation if (a) there are dysplastic features in the deep aspects and (b) the features of high-grade dysplasia (see below) are lacking.
 FIGURE 1.17 Barrett esophagus, low-grade dysplasia. The superficial epithelium shows mucin loss, nuclear enlargement, and hyperchromasia. There is no loss of nuclear polarity. |
extend to the surface. Loss of nuclear polarity is seen in high-grade dysplasia. Mitoses are readily identifiable. Inflammation is typically minimal. There is some evidence to suggest that high-grade dysplasia, accompanied by an ulcer, is a worrisome feature for an associated, unsampled, invasive carcinoma, and we suggest additional biopsies and/or endoscopic ultrasound when we see this pattern (27).
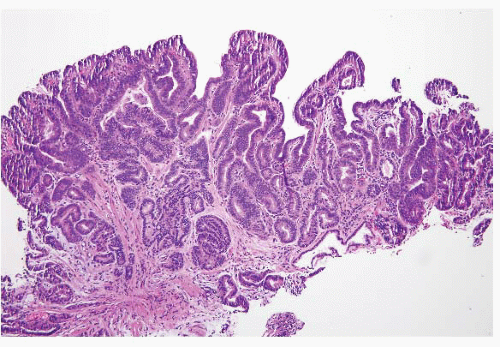 FIGURE 1.19 Barrett esophagus, high-grade dysplasia. This low power magnification shows total lack of surface maturation with crowding of cytologically abnormal glands. |
necrotic debris, (c) associated ulceration (e-Fig. 1.69), (d) neutrophils within dysplastic glands (Fig. 1.22), and (e) extension of neoplastic cells into the overlying squamous epithelium in a Pagetoid pattern. Zhu and colleagues demonstrated that identification of one of these features in association with high-grade dysplasia is associated with carcinoma in a subsequent resection
specimen in 39% of the cases. This figure increased to over 80% when two or more of these findings were present. In contrast, cases lacking any of these findings had no carcinoma in the resection specimen. Involvement of the overlying squamous epithelium with neoplastic glands in a Pagetoid pattern was associated with cancer in 100% of the cases (37). Other than Pagetoid spread pattern, these features, in fact, overlap with features of intramucosal carcinoma. Likewise, identification of single Paget cells (intraepithelial glandular neoplastic cells) in a biopsy specimen is invariably associated with an underlying adenocarcinoma containing at least a focal, poorly differentiated component (e-Figs. 1.85-1.87) (38). Overall, we believe that sufficient cytologic alterations “trump” architectural pattern.
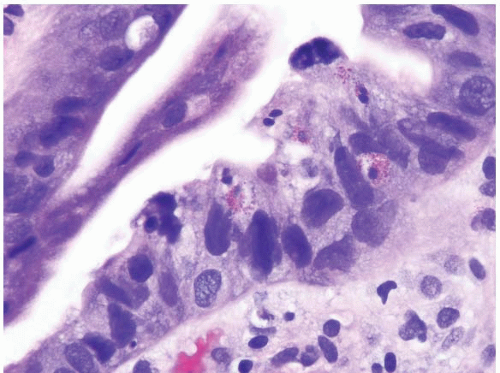 FIGURE 1.21 Barrett esophagus, high-grade dysplasia. This high power magnification shows loss of nuclear polarity and smudged, irregular, large nuclei. |
 FIGURE 1.22 Barrett esophagus, high-grade dysplasia. Luminal neutrophils located within glands with high-grade dysplasia should provoke concern for an associated, unsampled carcinoma. |
gastrointestinal tract, invasion into the lamina propria is more significant than in the colon, because colon lamina propria lacks significant lymphatic access. In the colon, invasion into the lamina propria is biologically equivalent to high-grade dysplasia, whereas in the esophagus, invasion into the
lamina propria can lead to metastatic disease (e-Figs. 1.102, 1.108, and 1.109). Interobserver variability can be a factor when diagnosing intramucosal carcinoma in a small biopsy (43), but this distinction is less important than it was in the past since both high-grade dysplasia and intramucosal carcinoma can be managed endoscopically (5,16).
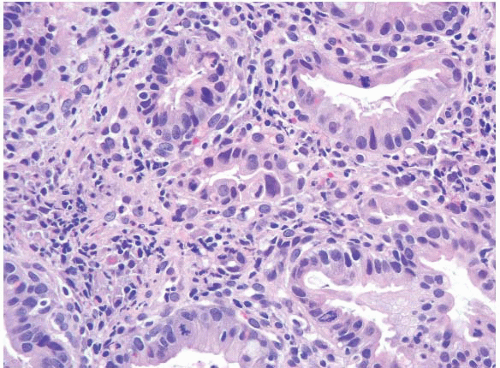 FIGURE 1.26 Intramucosal carcinoma. Note small clusters of epithelial cells within the lamina propria in association with glands showing high-grade dysplasia. |
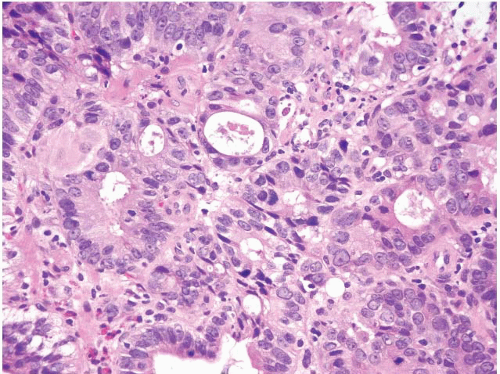 FIGURE 1.27 Intramucosal carcinoma. Note prominent nucleoli, back-to-back glands, and intraluminal debris. |
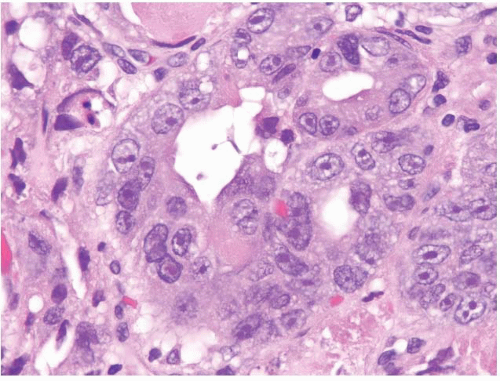 FIGURE 1.28 Intramucosal carcinoma. Higher magnification of Figure 1.27. Note the prominent nucleoli. |
 FIGURE 1.29 Invasive carcinoma. In carcinoma that has invaded into the submucosa, desmoplasia and a clearly infiltrative growth pattern may become more readily apparent, as in this case. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree