Fig. 42.1
Graphical presentation of the activity courses for 600 patients during 3–7 years after diagnosis. Adapted with permission from Elsevier [22]
Investigations to uncover clinical predictors of long-term disease course have been unsuccessful. Ritchie et al. found that there was no relationship between future disease course and disease extent or severity at the first attack. However, there was an association between disease extent during an attack year and maximal disease severity that year [18], which was confirmed later by Langholz et al. [22]. These findings indicate that severity and disease extent during an exacerbation are prognostic of only the immediately subsequent clinical course, without providing information on long-term disease. Thus, while our arsenal has expanded, a semi-prophylactic approach utilizing higher-intensity therapeutics in low disease activity states does not make sense when accounting for the potential risks of these drugs.
Colectomy rates are higher in the first year after diagnosis as clinicians attempt to subdue disease activity [19], but an active therapeutic approach can bring overall rates as low as 9 % by 10 years after diagnosis [23]. However, moderate attacks of colitis carry a 20 % colectomy rate, and severe attacks have colectomy rates as high as 47 % despite IV corticosteroids [24]. Extent of disease is also a factor in stratifying disease severity, with as many as 60 % of patients with pancolitis requiring surgery at 3 months [25]. However, unlike with CD, there is no accurate model to predict whether an individual with UC will progress to easily controlled intermittent flares or acute spikes in activity requiring surgery.
A Top-Down Strategy?
Certain differences must be underscored when explaining the divergence in approach to therapy for CD and UC. In CD, where clinical, serologic, genetic, and endoscopic data allow providers to forecast outcomes and surgical resection rates approach 80 % by 20 years after diagnosis, a top-down approach to inducing and maintaining remission has gained acceptance in clinical practice. The landmark ACCENT I study showed that CD patients who achieved mucosal healing with infliximab required fewer hospitalizations, surgeries, and ICU admissions [26]. This is logical given that unchecked inflammation will increase the complication rate and result in fistula or stricture formation [27]. To prevent these deleterious effects, a higher-intensity strategy aimed at suppressing inflammation has been successfully utilized in a subset of CD patients prone to a more severe disease outcome. However, this has not translated over to the treatment of UC, where disease course fluctuates as demonstrated in Fig. 42.1, and overall surgical rates are as low as 9 % at 10 years after diagnosis [23] and 20 % at 25 years of diagnosis [22]. With the established approach to therapy for UC, long-term survival rates equal those for age-matched controls, and there has been no difference from the general population in the capacity of UC patients to hold employment [19], with over 90 % of UC patients deemed fully capable of working after 10 years of diagnosis [22]. Since there is potential for a relatively benign disease course, adequate disease control is achievable with low-intensity therapy, and survival rates remain relatively high, our strategy in the treatment of UC continues to follow a step-up approach.
A Proactive, Step-Up Approach
Given our inability to identify and initiate early treatment in patients who will suffer from severe disease, focus should be shifted toward swiftly instituting targeted therapy based on early markers for disease activity. Thus, clinicians need not take a passive approach to treating disease flares and wait for symptoms to erupt. Validated clinical scores gauging disease severity combined with objective markers indicating degree of brewing inflammation can enable providers to take a proactive step-up approach to decide appropriate treatment intensity. Formal research evaluating the validity of these early disease markers as diagnostic and prognostic tools in IBD should be an area of future investigation.
How Is Disease Severity Best Measured?
Scoring scales have been validated to distinguish classes of disease severity. In 1955, Truelove and Witts published the first clinical criteria for classifying disease severity [28]. Mild disease was defined as fewer than four bowel movements per day with trace or no blood in the stool, without other signs of systemic toxicity, and a normal ESR. Severe disease was six or greater bloody stools daily, evidence of systemic toxicity (such as fever or tachycardia), anemia, and an elevated ESR. The American College of Gastroenterologists further clarified the definitions of moderate disease as cases with four stools daily with minimal signs of toxicity and fulminant disease for cases with more than ten bowel movements daily with continuous rectal bleeding, signs of systemic toxicity, abdominal tenderness or distension, anemia requiring transfusion, or colonic dilatation on imaging [2].
While symptom profile is the traditional measure to which therapy is titrated, mucosal healing has been implicated as a better gauge of long-term disease control and prognosis. Advances in endoscopy led to the inclusion of endoscopic appearance in parallel with clinical features by scales such as the UC-DAI and the Mayo score [29, 30]. One early study revealed that 40 % of patients who also achieved endoscopic remission after acute treatment versus 18 % of patients who still had endoscopic disease remained asymptomatic during the following year [31]. Increased inflammation has been correlated with higher colectomy and hospitalization rates in UC patients [32], and mucosal healing may help prevent disease extension and development of dysplasia. During an active flare, mucosal inflammation is often seen, and therapy is subsequently “stepped-up.” However, when evaluating the efficacy of maintenance therapy, we also propose that the presence of mucosal inflammation on endoscopy be a prompt for providers to consider “stepping-up” treatment intensity, especially in the presence of biomarker elevations which are outlined below.
What Are Early Markers of Disease Activity?
Laboratory markers, such as serum C-reactive protein (CRP), fecal calprotectin, and fecal lactoferrin, have been studied with the goal of providing measurable objective markers of disease activity and to avoid costly, invasive procedures.
Serum C-reactive protein (CRP) has been a useful tool for gauging disease activity, as its production is not altered by immunosuppressive medications, and its levels correlate with clinical severity and endoscopic inflammation [33–35]. A CRP level >12 mg/L has been suggested to be a marker of severe and extensive disease [36], and sustained elevation despite medications is indicative of inadequacy of therapy [35]. High CRP levels may be predictive of need for colectomy, as shown by an early study correlating CRP >45 mg/L with colectomy by 4 months [37, 38] and a later population-based study which found that persistent CRP levels >10 mg/L after 1 year of treatment with surgery by 4 years [39]. Because CRP is not affected by immunosuppressive medication, a decrease in its level is considered an objective marker of the effect of medical therapy on intestinal inflammation, even if there is no change in symptomatology [35].
Fecal calprotectin and fecal lactoferrin, two proteins involved in regulating the inflammatory process, are noninvasive, objective biomarkers for grading mucosal disease activity in IBD. Their potential diagnostic and prognostic utility have been studied, but due to lack of coverage for these tests by insurance companies, they are not commonly used, even in academic centers.
Fecal calprotectin is an iron-binding protein found primarily in neutrophils, and stool concentrations are being used as a surrogate marker for gut inflammation given its correlation with neutrophil incursion into the gut lumen [39]. Stool concentrations are increased in active UC, with a level greater than 50 mcg/g having over 79 % specificity and 91 % sensitivity for differentiating active versus inactive disease [39]. Stool calprotectin levels were found to correlate with degree of inflammation rather than disease extent [40], and concentrations in active UC patients were significantly higher than patients with inactive UC or control patients (402.16 mcg/g ± 48.0 vs. 35.93 mcg/g ± 3.39 vs. 11.5 mcg/g ± 3.42, p < 0.01) [39]. Additionally, a strong relationship between disease activity index (DAI) and fecal calprotectin was observed in the same cohort of patients (r = 0.866, p < 0.001), with calprotectin ranging from 100 to 500 mcg/g for DAI 6–9 and 500 to 1,000 mcg/g for DAI above 9 (more severe disease).
Fecal lactoferrin is another iron-binding protein secreted into the gut by activated neutrophils and is another surrogate for intestinal inflammation [41, 42]. It has been shown to be an accurate diagnostic tool for UC with a sensitivity of 92 % and specificity of 88 % [41] and can also be used to monitor disease activity. Lactoferrin concentrations are increased in active UC, with greater than 90 % correlation of levels to disease activity [42, 43]. Patients with active UC had significantly higher fecal lactoferrin levels than patients with inactive UC or control patients (median 51.1 mcg/mL, range 0–104 vs. 4.34 mcg/mL, 1–1,669 vs. 1.82 mcg/mL, 0–90) [44]. Patients with elevated fecal lactoferrin levels were also found to be prone to a disease flare when tapered off steroids [43]. These markers also have prognostic utility—with normalization of calprotectin predicting complete response to treatment and lactoferrin levels decreasing in correspondence to a decrease in Mayo score [45].
Findings of moderate-to-severe symptoms, endoscopic inflammation, or abnormal objective markers of inflammation, such as a persistently elevated fecal calprotectin >50 mg/kg, fecal lactoferrin, or serum CRP >12 mg/L despite 4 days of therapy, should prompt escalation to higher intensity of therapy.
Matching Treatment Intensity to Severity Scenarios
A primary goal of therapy is to “do no harm”—therefore providers must select medications for which the risk benefit ratio is balanced. Figure 42.2 shows our step-up model and breakdown of classes of therapeutic intensity. Patients with controlled disease on no medications or 5-ASAs via the topical or systemic route only are receiving a low-intensity treatment. While moderately active UC had been recognized as a clinically and endoscopically distinct category, its therapeutic algorithm had often been borrowed from mild or severe disease. A moderate-intensity regimen is indicated in those not adequately controlled on systemic or topical 5-ASAs and includes topical or systemic 5-ASAs in conjunction with corticosteroids or the addition of immunomodulators for those intolerant of corticosteroids or requiring greater than two courses of corticosteroids within 1 year. A high-intensity strategy would be reserved for those with persistent symptoms on moderate-intensity medications and includes anti-TNF agents, cyclosporine, or experimental drugs not currently indicated for the treatment of UC. All these therapies are associated with potentially toxic effects, and these risks must be weighed against the need to attain disease control in every individual.
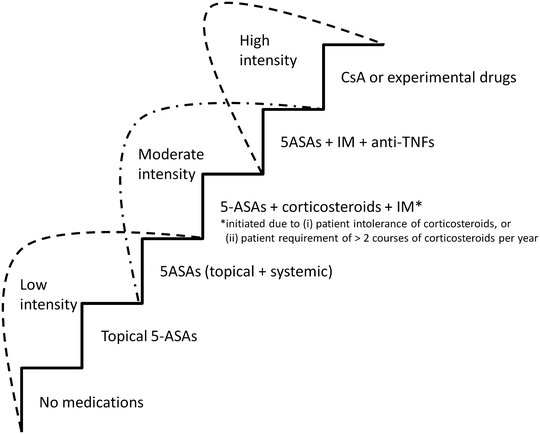

Fig. 42.2
A step-up algorithm matching treatment intensity to disease severity. 5-ASAs 5-aminosalicylates, IM immunomodulators, anti-TNFs anti-tumor necrosis factor agents, CsA cyclosporine
Low-Intensity Therapy: Topical and Systemic 5-ASAs
Systemic 5-ASAs
Aminosalicylates are the key to a low-intensity strategy to treatment of mildly active UC. Oral aminosalicylates are clearly effective at inducing therapeutic response in anywhere from 40 to 80 % of UC patients at 4 weeks, and their use as a first-line therapy in most UC patients has been widely accepted [2, 46, 47]. A recent 2012 Cochrane review investigated the ability of 5-ASAs versus placebo to induce remission in UC [48]. 5-ASA outperformed placebo for all endpoints—including adherence, adverse events, drug discontinuation, and clinical or endoscopic improvement—with 72 % of 5-ASA users versus 85 % of placebo patients failing to attain clinical remission (RR 0.86, 95 % confidence interval [CI] 0.81–0.91). There was also a trend toward 5-ASA superiority over sulfasalazine in inducing remission (RR 0.90, 95 % CI 0.77–1.04), and fewer 5-ASA patients had adverse events versus sulfasalazine users (15 % vs. 29 %, RR 0.48, 95 % CI 0.37–0.63).
After induction, mesalamine is used as maintenance therapy for UC [48–50]. A meta-analysis of mesalamine in the maintenance of remission in quiescent UC showed 5-ASA superiority over placebo, with 41 % of treatment versus 58 % of placebo patients suffering a relapse (RR 0.69, 95 % CI 0.62–0.77) [51]. The rate of adverse events between 5-ASA and placebo, different 5-ASA dosages, or various 5-ASA dosing strategies did not differ, and compliance rates were similar in all groups.
Although the above meta-analyses found no difference among various 5-ASAs, variations in dosing timing, delivery system, or volume reaching the colon are still believed to translate into some variation in clinical response [51]. Adherence to a regimen might be considered a more important factor than the 5-ASA formulation, as noncompliant patients are reported to have greater than a five times increased risk of recurrence (95 % CI 2.3–13, p < 0.001) [52, 53].
Topical 5-ASAs
Rectal 5-ASAs achieve higher levels of 5-ASA in the distal colon, and findings of higher rectal 5-ASA concentrations in patients without bloody stools than those with bloody stools suggest that maintaining high mucosal concentrations may be the factor determining efficacy of 5-ASAs [54]. 5-ASA enemas can also be used in combination with oral 5-ASAs to control symptoms in patients with extensive disease [55, 56]. Safdi et al. showed that combining 2.4 g of oral mesalamine with mesalamine enemas resulted in more rapid and complete symptom control in left-sided colitis [56]. Limitations to this mode of delivery include patient preference, although there is strong evidence to support its use in the presence of distal colitis.
Moderate-Intensity Therapy: 5 ASAs + Immunodilators, Steroids
5-ASAs
Higher doses lead to a higher mucosal concentration of 5-ASA, which is inversely related to endoscopic and histologic activity in UC (r = 0.712, p < 0.001) [57]. The ASCEND trials were randomized controlled phase 3 clinical trials that compared 4.8 g/day of mesalamine versus 2.4 g/day of mesalamine in achieving complete remission or response to therapy from baseline. Both ASCEND I and II demonstrated superiority of higher-dose mesalamine among patients with moderate UC, with a nonsignificant decrease in median time to achieving normal stool frequency and resolution of rectal bleeding [58, 59]. In ASCEND III, higher dose mesalamine was more effective in patients with difficult to treat disease who had previously required corticosteroids or multiple agents [60]. There was no difference in adverse events among the two dosing strategies [58–60].
The benefits of greater mesalamine doses in high-risk relapsers were further demonstrated by Frieri et al., where 18 patients had a decrease in recurrence episodes from 80 over 2 years to 8 over the following 2 years, after increasing the oral 5-ASA dose to 4.8 g daily and adding daily 5-ASA enemas [61]. There was a 100-fold increase in mucosal 5-ASA levels (median, 3–260 ng/mL) to which the authors attributed the striking clinical differences—over the first 2 years, there were 33 prescribed courses of corticosteroids, 93 hospital days, and 249 outpatient visits; this decreased to zero steroid courses or hospital days and 116 outpatient visits using higher-dose combination 5-ASA therapy. However, expecting patients to adhere to such a strict regimen is not realistic, and disease that is active despite systemic 5-ASAs often prompts consideration of higher-intensity strategies.
Initial treatment of active UC should be based on disease severity, with conventional 2.4 g/day dosing being effective in mild disease and higher doses providing incremental benefit for moderately active disease, especially those who have previously required corticosteroids or multiple agents to control symptoms.
Thiopurines
Thiopurines have long been used in Crohn’s disease [62], and this benefit has also been demonstrated in moderately active UC [63–65]. In 2008, a meta-analysis of thiopurines in UC found that azathioprine was superior to placebo, with a failure to maintain remission of OR 0.41 (95 % CI 0.24–0.70) [66]. A 2011 meta-analysis confirmed that azathioprine was effective for preventing relapse in quiescent UC (RR 0.60, 95 % CI 0.37–0.95) and showed a trend toward benefit in active UC (RR 0.85, 95 % CI 0.71–1.01) [67]. This outcome has been attributed the ability of thiopurines in achieving long-term mucosal healing in UC [68].
Ardizzone et al. conducted an RCT of azathioprine versus 5-ASA for steroid-dependent UC and found that 50 % of patients using azathioprine versus 35 % of 5-ASA treated-patients achieved clinical and endoscopic remission to a degree that allowed for discontinuation of steroid therapy [69]. At 6 months, clinical remission rates were similar, but the azathioprine group had a significantly higher rate of endoscopic remission. Additionally, the 5-ASA group also required an additional course of steroids to achieve these effects, and the authors of this study hypothesized that azathioprine’s ability to induce mucosal healing may be the reason for its efficacy.
Thiopurines are slow-acting drugs, and it can take up to 6 months to achieve a therapeutic response, thus limiting their utility in acute relapses. Use is further limited by the high rate of side effects, which typically manifest in the first month, and a significant toxicity profile [66]. Some dose-dependent toxicities include myelosuppression and hepatotoxicity; rarer dose-independent effects include pancreatitis, nausea, pneumonitis, and a slightly increased risk of lymphoma [66, 70].
Corticosteroids
Corticosteroids are successful at rapidly inducing remission for symptoms that persist or worsen despite medical therapy [28, 71], and their role has been firmly established for active UC. While immunologics and biologics expand our options against moderate-to-severe UC, steroids remain at the center of therapy given their established efficacy in inducing remission for moderate-to-severe disease. However, the metabolic, immunologic, and psychiatric side effects of corticosteroids stemming from supraphysiologic doses, lengthy courses, and withdrawal have resulted in more judicious use in mild-to-moderate disease [72]. Additionally, around 60 % of patients have an incomplete response to steroids [25, 71, 73], and a considerable proportion become steroid-dependent or fail to respond over time [24, 71]. While newer therapies have yet to achieve perfect success controlling IBD, they expand our arsenal against moderate-to-severe cases of UC. Unlike corticosteroids, these agents may more reliably induce mucosal healing, which has been shown to alter disease course [74, 75]. Newer therapeutic algorithms incorporating these agents are being developed with the goals of maximizing remission, minimizing corticosteroid dependence, and staving off colectomy.
High-Intensity Therapy: Anti-TNFs and Cyclosporine
Infliximab
The success of anti-TNF agents for the treatment of CD prompted investigation in UC patients, with particular interest on those nonresponders to conventional therapy [76]. Infliximab has since been approved to treat symptoms of colitis, induce and maintain clinical remission, and act as a steroid-sparing agent in moderately to severely active UC patients [24, 77].
The ACT 1 and 2 trials were randomized, double-blind, placebo-controlled studies evaluating infliximab for induction and maintenance of remission in UC patients who had failed immunosuppressives and/or corticosteroids [77]. The primary endpoint of both trials was a clinical response at 8 weeks, with secondary endpoints including clinical response at week 8 in steroid-refractory patients, discontinuation of steroids at week 30, and sustained clinical remission at week 30 in ACT-2 and week 54 in ACT-1. Almost one-third of the patients had steroid-refractory UC, defined as persistent symptoms despite an equivalent of ≥40 mg/day of prednisolone orally for two or more weeks or intravenously for one or more week. Clinical response was significantly greater with infliximab, and sub-analysis revealed preservation of this effect in steroid-refractory patients. In both trials, infliximab-induced clinical remission was associated with the discontinuation of corticosteroid use at week 30.
An extension study of the ACT-1 and -2 cohorts evaluated those patients who achieved benefit from infliximab and followed them for three subsequent years of therapy. The initial improvement in PGA score or IBDQ scores continued for the duration of therapy, without an increase in adverse events [78]. Infliximab was associated with a 50 % decrease in the hospitalizations, which has been hypothesized to result in increased HRQOL [79, 80]. A post hoc analysis of the ACT-1 and ACT-2 data found that maintenance with infliximab resulted in an absolute risk reduction of 7 % in colectomy rates [81], confirming findings in earlier pilot studies [24, 82]; however, it should be noted that patients thought to require colectomy within 12 weeks of enrollment were excluded from the ACT trials. A 2008 retrospective review of acute severe UC found that although the risk of urgent colectomy was decreased with infliximab use in steroid-refractory patients, there was actually no difference in long-term elective colectomy rates [83].
Infliximab can have serious adverse effects, but mucosal healing with infliximab can potentially lead to improved outcomes [84, 85]. Toxicities include risk of infection, which is potentiated by concomitant steroid use, skin eruptions, and less commonly malignancy and neurologic diseases [86]. There is the additional risk of antibody formation to infliximab from infusion reactions, serum-like sickness, and attenuation of response [87]. Infliximab is contraindicated in certain settings—with active infection, multiple sclerosis, severe heart failure, or a history of optic neuritis or malignancy [87]. Clinicians must weigh the risks of infliximab toxicity against the potential benefits on long-term outcomes.
Adalimumab
Given the success seen with infliximab in UC patients and adalimumab in CD patients, adalimumab was subsequently evaluated for the treatment of UC, with the rationale that anti-murine antibodies to infliximab would not influence the efficacy of a completely human anti-TNF monoclonal antibody. Anti-TNF-naïve and experienced UC patients were evaluated for short- and long-term response to adalimumab, with results indicating a role for adalimumab as a corticosteroid-sparing agent in UC [88] and long-term colectomy-free rates in these severely active UC patients reaching as high as to 59 % at 2 years [89].
The first randomized, double-blind placebo-controlled study evaluating adalimumab for induction and maintenance of remission in anti-TNF naïve patients with moderately to severely active UC who had failed immunosuppression found that adalimumab was safer and more effective than placebo in both induction of remission (19 % vs. 9 % at week 8) [90]. A second recently published trial, ULTRA 2, evaluated adalimumab in patients who had failed immunosuppressants or previous anti-TNF agents and showed that adalimumab was more effective in induction and maintaining remission, with response rates of 17 % vs. 9 % at weeks 8 and 52 [91]. Based on these results, adalimumab was recently approved for the treatment of moderately to severely active UC unresponsive to corticosteroids or thiopurines. Further studies evaluating its efficacy against currently used therapies have yet to be conducted.
Golimumab
Golimumab is a fully humanized monoclonal immunoglobulin also directed against TNF-α. Genetically engineered mice were immunized with human anti-TNFα resulting in an antibody with a human-derived variable and regions that are constant. The variable region of golimumab binds to both the soluble and transmembrane bioactive forms of TNF-α and as a result inhibits the biological activity of TNF-α. Golimumab has been shown in vitro to modulate the biological effects mediated by TNF including the expression of adhesion proteins responsible for leukocyte infiltration (E-selectin, ICAM-1, and VCAM-1) and the secretion of proinflammatory cytokines (IL-6, IL-8, G-CSF, and GM-CSF).
Golimumab has been approved by the Food and Drug Administration in the United States to treat moderately to severely active rheumatoid arthritis (RA), active psoriatic arthritis, active ankylosing spondylitis (AS), and recently gained regulatory approval in 2013 for the treatment of moderate-to-severe UC patients who have had an inadequate response or intolerance to prior conventional treatments or who require continuous steroid therapy. Golimumab is given subcutaneously, and for UC, the dosage recommended is 200 mg initially at week 0 and then 100 mg at week 2 and then 100 mg every 4 weeks.
A combined double-blind placebo-controlled phase 2 dose-finding and phase 3 dose-confirmation trials demonstrated golimumab’s efficacy for induction of a clinical response and remission in patients with moderate-to-severe ulcerative colitis (PURSUIT) [92, 93]. There were 1,064 adult patients with moderately to severely active UC (Mayo score: 6–12, endoscopy subscore ≥2). Patients were randomly assigned to groups given golimumab doses of 100 mg and then 50 mg (phase 2 only), 200 mg and then 100 mg, or 400 mg and then 200 mg, 2 weeks apart. The phase 3 primary endpoint was a clinical response at week 6. The secondary endpoints included clinical remission, mucosal healing, and IBDQ score change at week 6. In phase 2, median changes from baseline in the Mayo score were −1.0, −3.0, −2.0, and −3.0 in placebo and 100 mg/50 mg, 200 mg/100 mg, and 400 mg/200 mg golimumab respectively. In phase 3, rates of clinical response at week 6 were 51.8 % and 55 % among patients given 200 mg/100 mg and 400 mg/200 mg golimumab respectively vs. 29.7 % in the placebo group (p < 0.0001). Rates of clinical remission and mucosal healing and mean changes in the IBDQ scores were significantly greater in both the golimumab and placebo groups (p ≤ 0.0005).
In the phase 3, double-blind trial evaluating golimumab in the maintenance of a clinical response in patients with moderate-to-severe UC, patients who responded to the initial golimumab induction therapy were randomly assigned to groups given placebo or injections of 50 or 100 mg of golimumab every 4 weeks through week 52 [93]. Four hundred sixty-four patients were included in this study. Patients who responded to placebo in the induction study continued to receive placebo. Nonresponders in the induction study received 100 mg golimumab. The primary outcome was a clinical response maintained through week 54 and secondary outcomes included clinical remission and mucosal healing at week 30 and week 54. Clinical response was found to be maintained in 47.0 % receiving 50 mg golimumab, 49.7 % receiving 100 mg golimumab, and 31.2 % receiving placebo (p = 0.010 and p < 0.001 respectively). At weeks 30 and 54, 27.8 % patients who received 100 mg golimumab were in clinical remission and 42.4 % had mucosal healing compared to placebo (15.6 % and 26.6 %, p = 0.004 and p = 0.002, respectively) or 50 mg golimumab (23.2 % and 41.7 %).
Vedolizumab
Natalizumab, a humanized IgG4 monoclonal antibody directed against the a4 integrin adhesion molecule involved in endothelial leukocyte migration, was approved by the Food and Drug Administration (FDA) for the treatment of Crohn’s disease for both induction and maintenance of remission. Patient and physician concerns over its association with progressive multifocal leukoencephalopathy have led to a search for gut-specific anti-integrin action that would eliminate this risk. Drugs with selective effects in the alpha-4 beta-7 integrin and mucosal adhesion molecule (MadCAM-1) pathway were investigated. Vedolizumab, as a consequence of this pursuit, was born.
The results of the subsequent GEMINI studies investigating vedolizumab for both induction and maintenance in ulcerative colitis and Crohn’s disease were published in 2013. In the Crohn’s disease study, 368 patients were randomized to vedolizumab or placebo [94]. Disease activity was measured at week 6 by assessing the reduction of Crohn’s disease activity index (CDAI).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






