Fig. 34.1
Endoscopy of inflammatory and noninflammatory disorders of the pouch. (a) severe diffuse pouchitis, (b) cuffitis, (c) Crohn’s disease of the pouch—ulcers at the neo-terminal ileum, (d) pinhole pouch inlet stricture, (e) pouch anastomotic sinus, and (f) opening of cryptoglandular fistula at the dentate line
Although histology has a limited role in grading the degree of pouch inflammation, it provides valuable information on some special features, such as granulomas, viral inclusion bodies (for CMV infection), pyloric gland metaplasia (a sign of chronic mucosal inflammation) [48], neoplasia [49], ischemia, or prolapse. A diagnostic and treatment algorithm is proposed (Figs. 34.2 and 34.3).
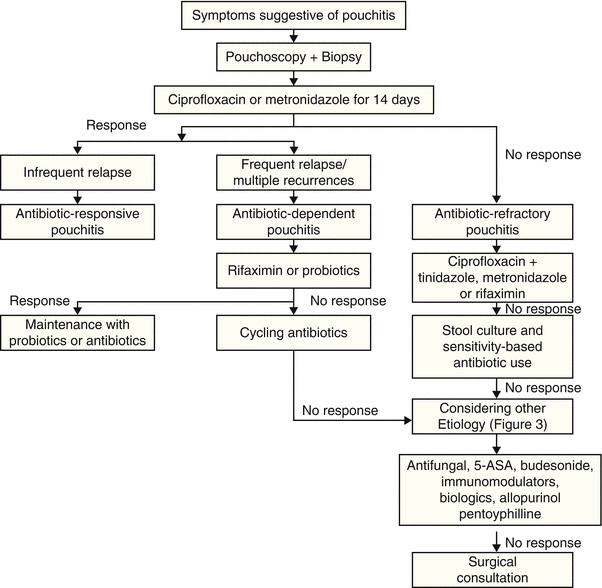
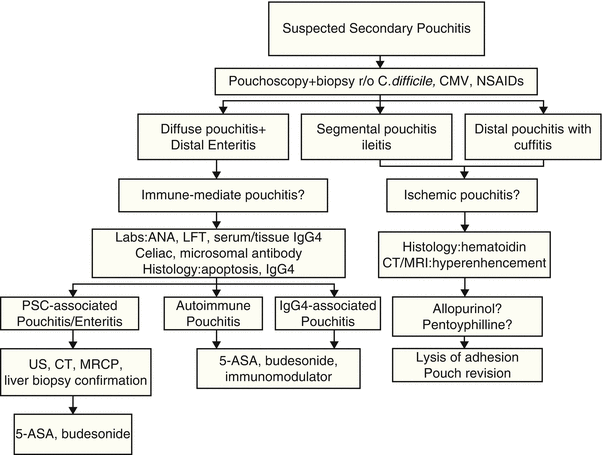

Fig. 34.2
Diagnostic and treatment algorithm of pouchitis

Fig. 34.3
Diagnostic and treatment algorithm of secondary pouchitis
Laboratory testing is often necessary as a part of the evaluation of patients with pouch disorders, particularly in patients with chronic pouchitis. In patients with persistent symptoms, celiac sprue serology, salicylate screening, and microbiological assays for Clostridium difficile and CMV infections should be performed [50]. Fecal assays of lactoferrin and calprotectin have been evaluated for the diagnosis and differential diagnosis of pouchitis. However, the use of laboratory tests may not replace pouch endoscopy as the first-line evaluation for the diagnosis and differential diagnosis of pouchitis.
The natural history of pouchitis is poorly defined. Pouchitis likely represents a disease spectrum from acute, antibiotic-responsive, bacteria-associated entity to chronic, antibiotic-refractory, immune-mediated entity. Based on the etiology, disease duration and activity, and response to medical therapy, pouchitis can be categorized into: (1) idiopathic vs. secondary (with etiology such as NSAID use and Clostridium difficile or CMV infection), (2) acute vs. chronic (with a cutoff of 4 weeks of persistent symptoms being defined as chronic pouchitis), (3) infrequent episodes vs. relapsing vs. continuous, and (4) responsive vs. refractory to antibiotic therapy [51]. Classification based on the response to antibiotic therapy is useful in clinical practice [52].
The various classification categories for pouchitis are noted in Table 34.1.
Table 34.1
Classification of pouchitis
Based on etiology | |
Idiopathic | With unidentified pathogens or triggering factors |
Secondary | • Clostridium difficile-associated • Cytomegalovirus-associated • Other pathogen-associated • NSAID-induced • Ischemic • Autoimmune or IgG4-associated • PSC-associated pouchitis/enteritis |
Based on duration of symptoms | |
Acute | Less than 4 weeks |
Chronic | Greater than 4 weeks |
Based on symptom pattern | |
Infrequent | <3 episodes per year |
Relapsing | ≥3 episodes per year or recurrence within 1 month of successful antibiotic therapy |
Based on response to antibiotic therapy | |
Antibiotic-responsive | Responds to a course of antibiotics |
Antibiotic-dependent | Requires ongoing antibiotic therapy to maintain response |
Antibiotic-refractory | Does not respond to a standard course of antibiotics |
We recently proposed a new disease category of pouchitis, namely, “autoimmune pouchopathy” [45]. The patients often presented with symptoms similar to “bacteria-associated” pouchitis, such as increased stool frequency, cramps, and urgency. On endoscopic examination, there was mucosal inflammation of the pouch body, with or without a long segment of inflammation in the afferent limb. Although there are currently no established diagnostic criteria, the diagnosis of “autoimmune pouchopathy” may be suspected if a patient has antibiotic-refractory pouchitis, concurrent autoimmune disorders (such as rheumatoid arthritis and Hashimoto’s thyroiditis), serum autoantibodies, and the presence of increased epithelial apoptosis (authors’ unpublished data).
We recently also described IgG4-associated pouchitis. We found that a subgroup of symptomatic pouch patients with concurrent autoimmune disorders had an increased number of IgG4-expressing plasma cells in the lamina propria of the pouch and/or afferent limb biopsies [53, 54]. On the other hand, we also found that the degree of tissue IgG4-expressing plasma cells on pouch biopsy did not necessarily correlate with the serum level of IgG4, a marker for autoimmune pancreatitis. In a separate study we demonstrated that a high-level serum IgG4 was also associated with chronic antibiotic-refractory pouchitis [55]. The description of the new disease entity of the pouch suggests that abnormal mucosal B-cell immunity may play a role in the disease process of pouchitis and potentially provide a new therapeutic target.
PSC was reported to be associated with not only pouchitis but also long-segment backwash ileitis [54]. Typically, pouch patients with concurrent PSC often had a long segment of enteritis, with an endoscopic and histologic pattern similar to that of diffuse pouchitis. We speculate that pouchitis in those patients may represent a separate disease entity of the pouch, i.e., PSC-associated pouchitis/enteritis.
Differential Diagnosis
There are overlaps in clinical presentations between a variety of inflammatory diseases of the pouch (such as pouchitis, cuffitis, and CD of the pouch) and some anatomic diseases (such as afferent limb or efferent limb obstruction, pouch sinus which is defined as a blind tract that may lead to an abscess cavity, and strictures).
Cuffitis occurs primarily in patients with a stapled pouch-anal anastomosis, in whom a segment of rectal mucosa is left in place and becomes inflamed [56, 57]. Cuffitis typically represents a recurrence of UC in the residual mucosa. However, other disease process may also contribute to the development of cuffitis, such as CD and ischemia [58]. Patients with cuffitis often present with bloody bowel movements, which seldom occur in conventional pouchitis.
One of the other common inflammatory disorders is CD of the pouch. CD of the pouch can occur after IPAA which is intentionally performed in a selected group of patients with CD with no small intestinal or perianal diseases [59]. CD is also inadvertently found in proctocolectomy specimens in patients with a preoperative diagnosis of UC or indeterminate colitis. However, a majority of patients with CD of the pouch were considered to develop the disease de novo. IPAA surgery with fecal stasis, sutures and anastomosis, ischemia, and re-routing of bowel may create a “CD-friendly” environment. Whether CD of the pouch or CD-like condition of the pouch is a true de novo, IBD is not known. Clinical phenotypes of CD of the pouch can be inflammatory, fibrostenotic, or fistulizing. The diagnosis of CD of the pouch often needs a combined assessment of symptoms, endoscopy, histology, radiography, and sometimes examination under anesthesia.
Patients with anatomic diseases (such as pouch leaks, sinus tracts, fistula, and abscesses) and pouch ischemia can present with symptoms resembling symptoms of patients with pouchitis. Again, pouch endoscopy is considered the first-line diagnostic modality. Additional evaluation with radiography or examination under general anesthesia may be helpful.
Irritable pouch syndrome is a functional disorder in patients with IPAA [60]. There are great overlaps in clinical presentation between irritable pouch syndrome and pouchitis. Currently, irritable pouch syndrome is a diagnosis of exclusion with symptoms but absence of endoscopic, radiographic, or histologic abnormalities. Pouch endoscopy is the diagnostic modality of choice for the distinction between pouchitis and irritable pouch syndrome.
Management
While acute pouchitis is easy to treat, chronic pouchitis remains difficult to manage. The management strategies vary based on the etiology, triggering factors, and classification of pouchitis (Figs. 34.2 and 34.3) [61].
Antibiotics
Since the majority of pouchitis is of bacterial etiology, antibiotic therapy is the mainstay of therapy. For antibiotic-responsive acute pouchitis, the first-line therapy includes a 14-day course of metronidazole (15–20 mg/kg/day) or ciprofloxacin (1,000 mg/day) [62, 63]. A randomized trial of ciprofloxacin (1,000 mg/day) and metronidazole (20 mg/kg/day) showed that patients treated with ciprofloxacin experienced a greater reduction in the disease activity scores and fewer adverse effects (0 % vs. 33 %) than those treated with metronidazole [63]. Most patients with acute pouchitis initially respond to ciprofloxacin at a dose of 500–1,000 mg/day or metronidazole at doses of 750–1,500 mg/day. Symptomatic improvement usually occurs within 1–2 days after initiation of therapy. Patients with relapsing or continuous pouchitis may require chronic maintenance ciprofloxacin therapy with doses ranging from 250 mg every third day up to 1,000 mg/day. Combination therapy with ciprofloxacin and metronidazole for 28 days was shown to be effective in treating backwash ileitis in a recent open-labeled trial [64]. Furthermore, diffuse pouchitis can be associated with backwash ileitis, particularly in patients with concurrent PSC. Those patients may be treated with antibiotics or oral budesonide (see below).
Rifaximin, a non-absorbed antibiotic frequently used in pouchitis, was not shown to be more effective than placebo in a small controlled trial [65]. However, rifaximin maintenance therapy appears to be effective in preventing relapse in a majority of patients with antibiotic-dependent pouchitis after induction of remission with a variety of antibiotics [66]. In this study, 51 patients began maintenance therapy with rifaximin (median dose 200 mg/day); 33 (65 %) maintained remission through 3 months. Of these 33 patients, 26 (79 %) successfully continued maintenance for 6 months after beginning maintenance, 19 (58 %) successfully continued for 12 months, and 2 (6 %) successfully continued for 24 months. Other oral antibiotics were also reported in open-labeled trials to be effective, including tetracycline, clarithromycin, amoxicillin/clavulanic acid, and doxycycline [67].
The management of chronic antibiotic-refractory pouchitis often poses a challenge. In fact, this phenotype of pouchitis is one of the most common causes for pouch failure requiring pouch excision or diversion. It is important to investigate contributing causes related to failure to antibiotic therapy. Fecal coliform sensitivity testing was shown to be helpful in guiding choice of appropriate antibiotics in patients with antibiotic-refractory pouchitis [68]. In this study, 80 % of patients achieved a clinical remission with individualized therapy based on sensitivity results.
For chronic antibiotic-refractory pouchitis, a combined use of antibiotic agents with a prolonged course may be attempted. In open-labeled trials, a combined therapy of ciprofloxacin (1,000 mg/day) with rifaximin (2,000 mg/day) [69, 70], metronidazole (1,000 mg/day) [71], or tinidazole (1,000–1,500 mg/day) for 4 weeks was reported to be effective [72]. However, maintenance of remission in this group of patients after a successful induction therapy with the dual antibiotic therapy remains to be challenging [73].
5-ASA Agents
There are no randomized trials comparing oral or topical 5-aminosalicylate (5-ASA) therapy with placebo in management of pouchitis. Anecdotal reports suggest that topical (enema) or oral mesalamine may be of benefit [76, 77]. Topical mesalamine is also reported to be of benefit in management of cuffitis [56]. Recently, a pilot, open-labeled study showed that oral sulfasalazine (3,000 mg/day) leads to pouchitis in remission in 63 % of patients [78]. Sulfasalazine may be particularly indicated in patients with concurrent pouchitis and arthralgia or arthropathy, as NSAID is normally contraindicated in patients with IPAA.
Corticosteroids
Oral prednisone is hardly used in the treatment of pouchitis due to its side effect profile and the lack of long-term efficacy. The only exception is pouchitis episode in pregnant women, in authors’ experience. On the other hand, oral and topically active budesonide has been used in both acute and chronic pouchitis. A randomized controlled trial budesonide enema was conducted in comparison with oral metronidazole [79]. Improvement was similar in both groups, but budesonide enemas had a more favorable side effect profile. Budesonide suppositories for 4 weeks were showed to result in endoscopic improvement or remission in patients with acute pouchitis, but six of ten (60 %) patients relapsed 8 weeks later [80]. For patients who fail antibiotics, oral budesonide may be an option. In an open-labeled study of 20 patients with chronic antibiotic-refractory pouchitis, oral budesonide (9 mg/day orally for 8 weeks) induced remission in 15 (75 %) patients [81]. In a separate open-labeled series, budesonide induced a 60 % response rate in patients with antibiotic-refractory pouchitis [82].
With the description of autoimmune pouchitis, IgG4-associated pouchitis, and PSC-associated pouchitis/enteritis, oral budesonide has been routinely used in the authors’ practice. Our anecdotal experience highlighted that some of the patients responded favorably to the therapy. We typically used oral budesonide 9 mg/day, with broken capsules, for treatment and 3–6 mg/day for maintenance therapy. As capsulated oral budesonide was designed for the pharmacologic action at the distal small bowel and autoimmune-associated pouchitis often involves whole small bowel and pouch, we expect that the administration of broken capsules may help drug delivery in a larger area of GI tract. Bone mineral density and blood glucose should be monitored in patients on long-term budesonide therapy.
Immunomodulators
There are scant data on immunomodulator therapy for pouchitis. For patients with chronic pouchitis who are dependent on long-term maintenance therapy with antibiotics or topical and/or oral steroids, immunomodulators such as azathioprine and 6-mercaptopurine may be an alternative. Our anecdotal experience suggests that 6-mercaptopurine may be beneficial for patients with autoimmune pouchitis or IgG4-associated pouchitis. Anecdotal reports also suggested the efficacy of calcineurin inhibitors such as cyclosporine [83] and tacrolimus for therapy of chronic pouchitis.
Biologics
Tumor necrosis factor alpha (TNF-α) expression has been shown significantly higher in mucosal pouch biopsies of patients with pouchitis than noninflamed pouches in UC patients [84]. Biological agents which have been routinely used in CD of the pouch have also been used in chronic refractory pouchitis [85]. Short-term treatment (10 weeks) with infliximab was reported to be effective in a small group (n = 10) of patients with chronic antibiotic-refractory pouchitis complicated with ileitis [86]. Clinical remission was achieved in nine patients, and endoscopic remission with a complete healing of all lesions was observed in eight patients. Infliximab was also reported to be effective on a long-term basis in patients with refractory luminal inflammation and in some patients whose disease is complicated with pouch fistulas [87]. In this retrospective study, after a median follow-up of 20 months, 56 % showed sustained clinical response while three out of seven fistula patients showed sustained fistula response.
Probiotics
It has been recommended by some individuals that patients with chronic pouchitis who achieve remission following antibiotic therapy but relapse more than three times per year should be treated with maintenance therapy [88]. Probiotics have been used as maintenance therapy for patients with antibiotic-dependent pouchitis or relapsing pouchitis (secondary prophylaxis), and also in prevention of pouchitis after IPAA surgery (primary prophylaxis). In addition, high-dose probiotics have been used for treating pouchitis. In a study of a probiotic agent, VSL#3®, 3,600 billion bacteria/day in treating mild pouchitis, 16 of 23 patients (69 %) were in remission after treatment [89]. A prospective, randomized, double-blind, placebo-controlled trial highlighted that treatment with VSL#3® at a dose of 900 billion bacteria/day was also effective in the prevention of the onset of acute pouchitis and improved quality of life of patients with IPAA [90]. A randomized trial of VSL#3® at a dose of 6 g/day was conducted for the maintenance therapy to prevent relapse of pouchitis, after remission was induced by oral ciprofloxacin (1,000 mg/day) plus rifaximin (2,000 mg/day). During the 9-month trial of 40 patients with relapsing pouchitis, 15 % in the probiotic group relapsed vs. 100 % in the placebo group relapsed [91]. A separate randomized trial of VSL#3® in patients with antibiotic-dependent pouchitis showed that 17 of 20 patients (85 %) in the VSL#3® group maintained clinical remission, compared to remission in 1 of 16 patients (6 %) in the placebo group [92]. A meta-analysis of five randomized, placebo-controlled clinical trials was performed. Pooling of the results from these trials yielded an odds ratio of 0.04 in the treatment group in comparison with the placebo group. The benefit of probiotics in the management of pouchitis after IPAA operation was confirmed by the meta-analysis [93]. However, the routine use of probiotics for the induction and maintenance therapy of pouchitis has stirred some controversy. Some post-market open-labeled studies reported a much lower response rate of pouchitis that was originally reported. The above outstanding results have been challenged by two recent post-market open-labeled trials. In a study of 31 patients with antibiotic-dependent pouchitis treated with VSL#3® for maintenance therapy after 2 weeks of treatment with ciprofloxacin, 25 patients (81 %) had stopped the agent at 8 months, mainly because of the lack of efficacy or development of adverse effects [94]. Similar results were reported in a separate open-labeled trial [95].
Other Agents
Other agents, including allopurinol [96, 97], bismuth carbomer enema [98, 99], short-chain fatty acid (SCFA) enemas, and glutamine enemas, have been reported to be of benefit from uncontrolled data. However, based on currently available controlled data, bismuth and allopurinol cannot be advocated as a therapy for pouchitis [100]. Considering the low overall response rates observed during open therapy with SCFA or glutamine, it appears not beneficial for pouchitis and cannot be advocated as standard therapy [100]. Recently, leukocytapheresis [101] and AST-120 (spherical carbon adsorbent) [102] showed benefit in patients with acute pouchitis. Randomized, placebo-controlled trials are warranted for assessing the long-term efficacy of these strategies.
Endoscopic Polypectomy
Chronic pouchitis can be associated with single or multiple, small or large inflammatory polyps. Large (>1 cm) pouch polyps can occasionally cause bleeding and can be dysplastic. Endoscopic polypectomy is feasible, which may be helpful in controlling patients’ symptoms, in conjunction with medical therapy [103].
Summary and Conclusions
Pouchitis is the most common long-term complication of IPAA, which represents a spectrum of disease processes with different clinical phenotypes, risk factors, pathogenetic pathways, natural history, and prognosis. Pouch endoscopy is the most valuable tool for diagnosis and differential diagnosis. While the majority of patients with pouchitis respond favorably to antibiotic therapy, antibiotic-dependent or antibiotic-refractory diseases have posed a therapeutic challenge. Management of pouchitis, particularly chronic pouchitis, can be difficult. The search for a secondary etiology of pouchitis, such as Clostridium difficile infection, should be performed. Medical treatment of pouchitis is largely empiric, and only a few small randomized, placebo-controlled trials have been conducted. To date, there were no FDA-approved agents for pouchitis or other pouch disorders. A multidisciplinary approach involving gastroenterologists and colorectal surgeons, together with a team of GI pathologists and GI radiologists is advocated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






