Fig. 8.1
Distribution of 5-ASA suppository in a patient with ulcerative proctitis. 99mTc-labeled 5-ASA suppository was given to a patient with refractory ulcerative proctitis. The medication spread to the sigmoid colon after 3.5 h in (a) anterior view and (b) right lateral view. From Williams CN, Haber G, Aquino JA. Double-blind, placebo-controlled evaluation of 5-ASA suppositories in active distal proctitis and measurement of extent of spread using 99m Tc-labeled 5-ASA suppositories. Dig Dis Sci. 1987;32(12 Suppl):71S–75S. With permission from Springer © 1987 [19]
Mesalamine foam is also useful in the treatment of left-sided ulcerative colitis. In a randomized, double-blind, placebo-controlled study of 2 g of mesalamine foam vs. placebo in 111 patients with mild to moderately active proctitis, proctosigmoiditis, or left-sided ulcerative colitis over a 6-week period, 65 % in the treatment group versus 40 % in the placebo group achieved clinical remission, 57 % vs. 37 % achieved endoscopic remission, and 59 % vs. 41 % had improved histologic indices [21]. The treatment group utilized two 1 g 5-ASA foam enemas. In a subgroup analysis, the clinical benefit of treatment was seen in the group with mild disease (with a clinical disease activity index of eight or less), but was not seen in the smaller group of 16 patients with moderate disease activity. In terms of disease location, patients with proctosigmoiditis had the highest response rate to treatment (61 %), as compared to those with proctitis (54 %) or left-sided colitis (40 %).
A crossover study of 10 patients compared a 99mTc-labeled preparation of 4 g 5-ASA in 20 mL foam vs. 4 g 5-ASA in 100 mL suspension enemas [22]. In vitro studies of the foam suggested that the 4 g foam expanded to 180–200 mL after being expelled with a propellant gas. Study subjects were asked to lie supine for 4 h after administration. After 120 min, 6/10 patients receiving the 5-ASA foam had homogenous coverage of the medication in the descending colon, 3/10 had nonhomogenous spread in the descending colon, and 1/10 had no drug coverage in the descending colon. In the 5-ASA enema group, 7/10 had nonhomogenous coverage in the descending colon, while 3/10 had no coverage.
The total foam volume does not appear to determine efficacy. A 2007 study by Eliakim et al. reported remission rates of 77 % after 6 weeks of twice-daily treatment with either a 30 mL or a 60 mL 1 g mesalamine foam [23].
A gel formulation of mesalamine has also been designed with the hope for efficacy with easier tolerability and less “messy” application. Unlike the mesalamine foam, a propellant gas is not instilled into the colon with the gel formulation. A 4 g/60 mL 5-ASA gel was tested in a study of 12 patients with mild to moderately active ulcerative colitis [24]. In this open-label study, patients were asked to lie on their left side for 4 h after administration of a 99mTc-labeled gel. 11/12 (92 %) of patients had spread of the gel beyond the sigmoid colon, while 6/12 (50 %) had coverage past the splenic flexure [24]. In a randomized, multicenter, investigator-blind trial of 103 patients with mild to moderate left-sided ulcerative colitis or proctosigmoiditis, the efficacy of a 2 g/60 mL 5-ASA gel enema was compared to a 2 g/120 mL 5-ASA foam enema over a 4-week treatment period [25]. After 4 weeks of therapy, clinical remission was observed in 76 % of patients using the gel and 69 % using the foam. Endoscopic remission was achieved in 51 % using the gel and 52 % using the foam. Patients using the foam reported more difficulty in retention (25 % vs. 6 %), more bloating (50 % vs. 26 %), and more discomfort (48 % vs. 26 %) [25].
The distribution of all of the topical mesalamine formulations is variable and is partially dependent on patient factors, such as severity of inflammation and ability to lie on their left side for an extended time after administration. In general, suppositories are expected to reach the rectum, foams are expected to reach the rectum and sigmoid, and gel and liquid enemas can reach the splenic flexure (Fig. 8.2).

Fig. 8.2
Expected distribution of topical mesalamine formulations. (a) Suppositories reach the rectum. (b) Foam reaches the rectum and sigmoid colon. (c) Liquid and gel enemas can reach the splenic flexure. From Marshall JK, Irvine EJ. Putting rectal 5-aminosalicylic acid in its place: The role in distal ulcerative colitis. Am J Gastroenterol. 2000;95(7):1628–1636 [57]
Topical 5-ASA for Induction of Left-Sided Ulcerative Colitis Remission
Marshall et al. published a Cochrane Database systematic review of rectal 5-aminosalicylic acid for induction of remission in ulcerative colitis, evaluating randomized trials comparing rectal 5-ASA to placebo or another active therapy in patients with ulcerative colitis with a distal disease margin 60 cm from the anal verge or distal to the splenic flexure [26]. The authors conducted a search of the MEDLINE database (1966–2008), the Cochrane Central Register of Controlled Trials and the Cochrane IBD/FBD Group Specialized Trials Register, as well as manual reviews of reference listings and conference proceedings. Thirty-eight trials fulfilled the inclusion criteria. This group found that topical 5-ASA was superior to placebo for inducing remission, with a pooled odds ratio for eight trials of 8.30 for symptomatic remission (95 % CI 4.28–16.12, p < 0.00001). The pooled odds ratio for seven of the same eight trials for endoscopic remission was 5.31 (95 % CI 3.15–8.92, p < 0.00001). Furthermore, the pooled odds ratio for histologic remission was 6.28 (5 trials, 95 % CI 2.74–14.40; p < 0.0001) (Table 8.1).
Table 8.1
Summary of results from trials included in the Marshall et al. meta-analyses of studies comparing rectal 5-ASA to placebo for clinical remission, endoscopic remission, and histologic remission [26]
Study | Medication | Disease location | Duration | Remission or response and data |
|---|---|---|---|---|
Campieri et al. [28] | 1 and 1.5 g 5-ASA suppositories vs. placebo | Less than 20 cm from the anal verge | 4 Weeks | Clinical remission: 69 % 5-ASA 1 g/day vs. 74 % 5-ASA 1.5 g/day vs. 39 % placebo |
Endoscopic remission: 55 % 5-ASA 1 g/day vs. 59 % 5-ASA 1.5 g/day vs. 23 % placebo | ||||
Histologic remission: 10 % 5-ASA 1 g/day vs. 16 % 5-ASA 1.5 g/day vs. 6 % placebo | ||||
Campieri et al. [30] | 1.5 g 5-ASA suppositories vs. placebo | Less than 20 cm from the anal verge | 30 Days | Clinical remission: 56 % 5-ASA vs. 7 % placebo |
Endoscopic remission: 41 % 5-ASA vs. 7 % placebo | ||||
Histologic remission: 28 % 5-ASA vs. 3 % placebo | ||||
Campieri et al. [59] | Sucralfate 10 g vs. 5-ASA 2 g vs. placebo in 100 mL enemas | Mild to moderate activity no further than splenic flexure | 30 Days | Clinical improvement: 22 % sucralfate vs. 94 % 5-ASA vs. 14 % placebo |
Endoscopic improvement: 22 % sucralfate vs. 88 % 5-ASA vs. 14 % placebo | ||||
Histologic improvement: 17 % sucralfate vs. 83 % 5-ASA vs. 7 % placebo | ||||
Campieri et al. [60] | 1, 2, and 4 g 5-ASA enemas vs. placebo | Mild to moderate UC distal to the splenic flexure | 4 Weeks | Clinical remission: 85 % 5-ASA 1 g vs. 83 % 5-ASA 2 g vs. 86 % 5-ASA 4 g vs. 41 % placebo |
Endoscopic remission: 74 % 5-ASA 1 g vs. 73 % 5-ASA 2 g vs. 79 % 5-ASA 4 g vs. 30 % placebo | ||||
Histologic remission: 63 % 5-ASA 1 g vs. 70 % 5-ASA 2 g vs. 76 % 5-ASA 4 g vs. 15 % placebo | ||||
Hanauer et al. [58] | 1, 2, or 4 g 5-ASA enema vs. placebo | Mild to moderate UC extending less than 30 cm from the anal verge | 8 Weeks | Clinical remission: vs. 47 % 5-ASA 1 g vs. 49 % 5-ASA 2 g vs. 44 % 5-ASA 4 g vs. 14 % placebo |
Endoscopic remission: 59 % 5-ASA 1 g vs. 65 % 5-ASA 2 g vs. 66 % 5-ASA 4 g vs. 24 % placebo | ||||
Histologic remission: 42 % 5-ASA 1 g vs. 49 % 5-ASA 2 g vs. 55 % 5-ASA 4 g vs. 16 % placebo | ||||
Moller et al. [61] | 3 g sulfasalazine enema vs. placebo | Extending less than 15 cm from the anal verge | 2 Weeks | Symptomatic response: 81 % excellent response (no symptoms) with sulfasalazine vs. 14 % excellent response with placebo |
Endoscopic response: 75 % excellent response (full endoscopic remission) with sulfasalazine vs. 21 % excellent response with placebo | ||||
Pokrotneiks et al. [21] | 2 g 5-ASA foam enema vs. placebo | Mild to moderate UC distal to the splenic flexure | 6 Weeks | Clinical remission: 65 % 5-ASA vs. 40 % placebo |
Endoscopic remission: 57 % 5-ASA vs. 37 % placebo | ||||
Histologic improvement: 59 % 5-ASA vs. 41 % placebo | ||||
Williams et al. [19] | 5-ASA suppositories 500 mg TID vs. placebo | Extending less than 15 cm from the anal verge | 6 Weeks | Mean DAI treatment group was 0.4 ± 0.9 compared to 5.4 ± 3.4 in the placebo |
Remission achieved by 79 % on 5-ASA compared to 8 % placebo |
Our group at the University of Chicago conducted a meta-analysis and overview of the mesalamine literature from 1958 to 1997 on treatment options for left-sided ulcerative colitis and ulcerative proctitis [27]. This overview reported that in placebo-controlled trials studying mesalamine enemas in active left-sided ulcerative colitis, mesalamine enemas showed a duration, but not dose response, in achieving remission.
In the University of Chicago study, meta-analyses were conducted for studies examining mesalamine suppository treatment for ulcerative proctitis. One of these meta-analyses included two studies of 1 g mesalamine suppositories examining clinical and endoscopic remission at 2 weeks [28, 29]. Meta-analysis of these two studies showed a pooled advantage of mesalamine suppositories over placebo of 23.6 % for clinical remission with the 95 % confidence interval crossing zero (−8.8. to 56.0). The pooled advantage for endoscopic remission was 32.7 % with a CI of 15.3–50.1. The University of Chicago group performed a separate meta-analysis of two studies of 1.5 g mesalamine suppositories for 4 weeks and two studies of 1.5 g mesalamine suppositories for 6 weeks, which showed a pooled advantage over placebo in inducing clinical remission of 44.3 % (CI 29.4–59.1) at 4 weeks and 57.9 % (CI 36.1–79.7) at 6 weeks [19, 28, 30, 31]. The pooled advantage in achieving endoscopic remission at 4 weeks was 33.3 % higher in the treatment group (CI 18.5–48) and the pooled advantage over placebo in achieving clinical remission at 4 weeks was 52 % (CI 37.8–66.3). Overall review of mesalamine suppository studies for ulcerative proctitis revealed a duration, but not dose response (Fig. 8.3).
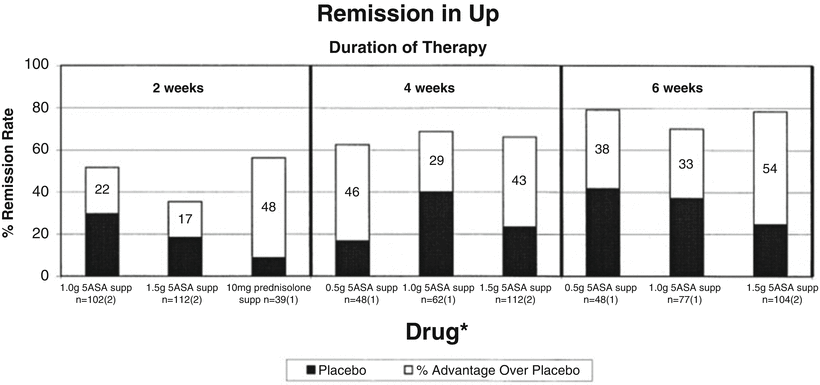

Fig. 8.3
Comparison of mesalamine suppositories for treatment of ulcerative proctitis. Numbers within bar graphs are percent advantage over placebo. N is the number of patients; the number of studies is in parentheses. From Cohen RD, Woseth DM, Thisted RA, Hanauer SB. A Meta-analysis and Overview of the Literature on Treatment Options for Left-Sided Ulcerative Colitis and Ulcerative Proctitis. Am J Gastroenterol. 2000;95(5):1263–1276 [27]
Comparison of Topical Mesalamine to Topical Corticosteroids
The Marshall et al. 2010 meta-analysis included a study of rectal 5-ASA as compared to rectal corticosteroids [26]. This analysis showed that rectal 5-ASA was superior to rectal corticosteroids for inducing symptomatic remission. A comparison of six trials showed a pooled odds ratio of 1.65 (95 % CI 1.11–2.45, p = 0.01) for symptomatic remission.
Lee et al. compared 2 g 5-ASA foam to 20 mg prednisolone foam in a 4-week trial of 295 patients in 36 centers in the United Kingdom with mild to moderately active UC [32]. The 5-ASA group achieved a significantly higher rate of clinical remission than the prednisolone group (52 % vs. 31 %, p < 0.001). The rates of endoscopic and histologic remission did not differ between groups. In a separate study, the Danish 5-ASA group reported a double-blind, multicenter trial of 1 g/day 5-ASA enemas compared to 25 mg/day prednisolone enemas for 4 weeks in 123 patients [33]. The overall response to therapy was defined as the sum of the clinical and endoscopic effects. Though no significant difference in clinical and endoscopic improvement occurred in the groups after 2 weeks, rates of remission did significantly differ, with 51 % in the 5-ASA group and 31 % in the prednisolone group achieving remission (p < 0.05).
Two studies compared 5-ASA foam or enema to beclomethasone foam or enema [34, 35]. Biancone et al. found, in a randomized, multicenter, double-blind trial, that 3 mg beclomethasone dipropionate foam or enema vs. 2 g 5-ASA foam or enema did not have significantly different rates of remission (24 % vs. 28 % at 4 weeks and 36 % vs. 52 % at 8 weeks) [34]. Gionchetti et al. also reported on a single-center, randomized, investigator-blind trial which demonstrated that patients with active distal UC receiving 1 g 5-ASA enemas or 3 mg beclomethasone propionate enemas for 6 weeks both had significantly improved disease activity indices but no statistically significant difference in the rates of clinical remission [35].
Farup et al. compared 5-ASA suppositories 500 mg twice daily to hydrocortisone foam 178 mg twice daily for 4 weeks [36]. Remission rates at 2 and 4 weeks did not significantly differ between treatments. A nonsignificant trend towards more histologic improvement with 5-ASA suppositories at 2 and 4 weeks (70 and 78 % vs. 50 and 61 %, respectively) was noted. Patients on 5-ASA suppositories had a greater mean increase in DAI than those on hydrocortisone foam, which was attributed to improved efficacy in the subgroup with proctitis.
Finally, Leman et al. compared 1 g 5-ASA enemas to 2.3 mg budesonide enemas in 97 patients with UC distal to the splenic flexure at endoscopy [37]. Clinical remission was achieved in 60 % of the 5-ASA patients vs. 38 % of the budesonide patients (p = 0.03), although there was no statistical difference in the rates of endoscopic improvement, histologic improvement, or histologic remission between the two groups.
Efficacy of Topical vs. Oral Mesalamine in Achieving Remission
Ford et al. have recently performed a systematic review and meta-analysis examining the efficacy of oral vs. topical, or combined oral and topical 5-ASA in ulcerative colitis [38]. Four randomized, controlled trials with endpoints between 4 and 8 weeks compared topical 5-ASA to oral 5-ASA for induction of remission in mild to moderate UC [39–42]. One trial, by Gionchetti et al., studied only patients with proctitis on 400 mg 5-ASA suppositories three times daily, while the other three studies examined the use of 4 g 5-ASA enemas. Overall, 49.5 % of patients taking topical 5-ASA alone failed to achieve remission compared to 58.7 % of patients on oral 5-ASA alone. The relative risk of failure to achieve remission with topical 5-ASA was 0.82 (95 % CI = 0.52–1.28). Statistically significant heterogeneity between studies was noted. When the Gionchetti study of patients with proctitis was removed from the analysis, the relative risk of remission with topical vs. oral 5-ASA was 1.04 (95 % CI 0.79–1.37).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






