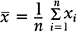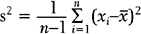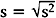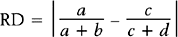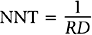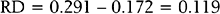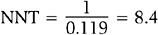Chapter 9 Statistics for the Clinical Scientist
LEVEL OF MEASUREMENT AND VARIABLE STRUCTURE
Variable Structure
A dependent variable (also called outcome, endpoint, or response variable) is the primary variable of a research study. It is the behavior of such a variable that is of primary concern, including the effects (or influence) of other variables on it. An independent variable (also called explanatory, regressor, or predictor variable) is the variable whose effect on the dependent variable is being studied. Designation of which variables are dependent and which are independent in a research study establishes the variable structure. How a variable is handled statistically depends in large part on its level of measurement and its variable structure.1
EXPERIMENTAL/RESEARCH DESIGN
The three core principles of experimental design are (1) randomization, (2) replication, and (3) control or blocking. Random selection of subjects from the study population or random assignment of subjects to treatment groups is necessary to avoid bias in the results. A random sample of size n from a population is a sample whose likelihood of having been selected is the same as any other sample of size n. Such a sample is said to be representative of the population. See any standard statistics text for a discussion of how to randomize.2,3
Completely Randomized Design (CRD)
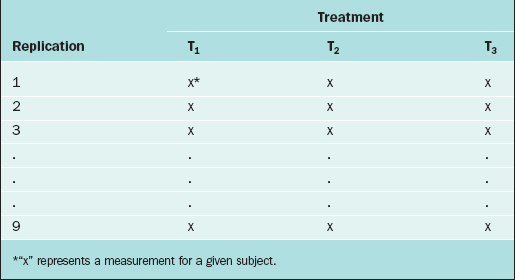
In the CRD, subjects are randomly assigned to experimental groups. As an example, suppose that the thickness of the endometrium is observed via ultrasound for women randomly assigned to three different fertility treatments (treatments T1, T2, and T3). Twenty-seven subjects are available for study, so 9 subjects are randomly selected to receive treatment T1, 9 are randomly selected from the remaining 18 to receive treatment T2, and the remaining 9 receive treatment T3. The data layout appears in Table 9-1.
Randomized Complete Block Design (RCBD)
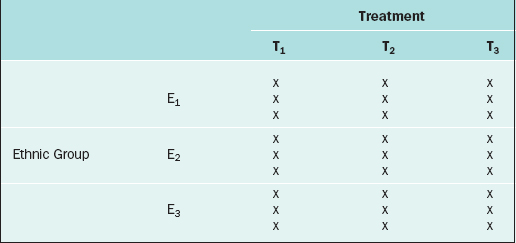
The RCBD is a generalization of the CRD whereby a second factor is included in the design so that the comparison of the levels of the treatment factor can be adjusted for its effects. For example, in the endometrial thickness study, there may be three different ethnic groups of women, E1, E2, and E3, included in the study. Because the variation of the endometrial thicknesses may be higher between two different ethnic groups than within a given ethnic group, it would be wise to block on the ethnic group factor. In this case, we would randomly select 9 subjects from ethnic group E1 and randomly assign 3 to each of the three treatments; likewise for ethnic groups E2 and E3, for a total of 27 subjects. The data layout would appear as in Table 9-2.
Repeated Measures Design
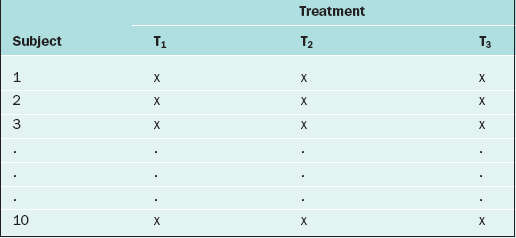
If in the RCBD the blocking factor is the subject, and the same subject is measured repeatedly, then the design becomes a repeated measures design. For instance, suppose that each of 10 subjects receives three different BP medications at different time points. For each medication, the BP is measured 5 days after the medication is started, then there is a 2-week “wash-out period” before starting the next medication. Also, the order of the medications is randomized. The data layout appears as in Table 9-3.
Many more sophisticated and complex experimental designs exist, and can be found in any standard text on experimental design.4,5 For evaluating the validity, importance, and relevance of clinical trial results, see the article by The Practice Committee of the American Society for Reproductive Medicine.6 For surveys, there are many ways in which the sampling can be carried out (e.g., simple random sampling, stratified random sampling, cluster sampling, systematic sampling, or sequential sampling).7,8
DESCRIPTIVE STATISTICAL METHODS
Continuous Variables
An important numerical measure of the centrality of a continuous variable is the mean:
where n = number of observations and xi = ith measurement in the sample.
Note that the variance is approximately the average of the squared deviations (or distances) between each measurement and its mean. The more dispersed the measurements are, the larger s2 is. Because s2 is measured in the square of the units of the actual measurements, sometimes the standard deviation is used as the preferred measure of variability:
Also of interest for a continuous variable is its distribution (i.e., a representation of the frequency of each measurement or intervals of measurements). There are many forms for the graphical display of the distribution of a continuous variable, such as a stem-and-leaf plot, boxplot, or bar chart. From such a display, the central location, dispersion, and indication of “rare” measurements (low frequency) and “common” measurements (high frequency) can be identified visually.2
It is often of interest to know if two continuous variables are linearly related to one another. The Pearson correlation coefficient, r, is used to determine this. The values of r range from –1 to +1. If r is close to –1, then the two variables have a strong negative correlation (i.e., as the value of one variable goes up, the value of the other tends to go down [consider “number of years in practice after residency” and “risk of errors in surgery”]). If r is close to +1, then the two variables are positively correlated (e.g., “fetal humeral soft tissue thickness” and “gestational age”). If r is close to zero, then the two variables are not linearly related. One set of guidelines for interpreting r in medical studies is: |r| > 0.5 ⇒ “strong linear relationship,” 0.3< |r| ≤ 0.5⇒ “moderate linear relationship,” 0.1 < |r| ≤ 0.3⇒ “weak linear relationship,” and |r| ≤ 0.1⇒ “no linear relationship”.9,10
Discrete Variables
For two discrete variables the data are summarized in a contingency table. A two-way contingency table is a cross-classification of subjects according to the levels of each of the two discrete variables. An example of a contingency table is given in Table 9-4.
Table 9-4 Contingency Table for the Cross-classification of Subjects According to Treatment and Outcome.
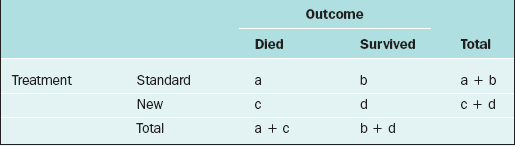
Here, there are a + b + c + d subjects in the sample; there are “a” subjects under the standard treatment who died, “b” subjects under the standard treatment who survived, and so on. If it is desired to know if two discrete variables are associated with one another, then a measure of association must be used. Which measure of association would be appropriate for a given situation depends on whether the variables are nominal or ordinal or a mixture.11,12
In medical research, two of the important measures that characterize the relationship between two discrete variables are the risk ratio and the odds ratio. The risk of an event, p, is simply the probability or likelihood that the event occurs. The odds of an event is defined as p/(1–p) and is an alternative measure of how often an event occurs. The risk ratio (sometimes called relative risk) and odds ratio are simply ratios of the risks and odds, respectively, of an event for two different conditions. In terms of the contingency table given in Table 9-4, these terms are defined as follows.
| Term | Definition |
|---|---|
| risk of death for those on the standard treatment | a/(a+b) |
| risk of death for those on the new treatment | c/(c+d) |
| odds of death for those on the standard treatment | a/b |
| odds of death for those on the new treatment | c/d |
| risk ratio of death | a(c+d)/c(a+b) |
| odds ratio of death | ad/bc |
The inverse of the risk difference is the number needed to treat, NNT:
NNT is an estimate of how many subjects would need to receive the new treatment before there would be one more or less death, as compared to the standard treatment.
For example, in a study by Marcoux and colleagues13 341 women with endometriosis-associated subfertility were randomized into two groups, laparoscopic ablation and laparoscopy alone. The study outcome was “pregnancy > 20 weeks’ gestation.” Of the 172 women in the first group, 50 became pregnant; of the 169 women in the second group, 29 became pregnant (a = 50, b = 122, c = 29, and d = 140). The risk of pregnancy is 0.291 in the first group and 0.172 in the second group; the corresponding odds of pregnancy are 0.410 and 0.207. The risk ratio is 1.69 and the odds ratio is 1.98. The likelihood of pregnancy is 69% higher after ablation than after laparoscopy alone; the odds of pregnancy are about doubled after ablation compared to laparoscopy alone. The risk difference is
and number needed to treat is
Rounding upward, approximately 9 women must undergo ablation to achieve 1 additional pregnancy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree