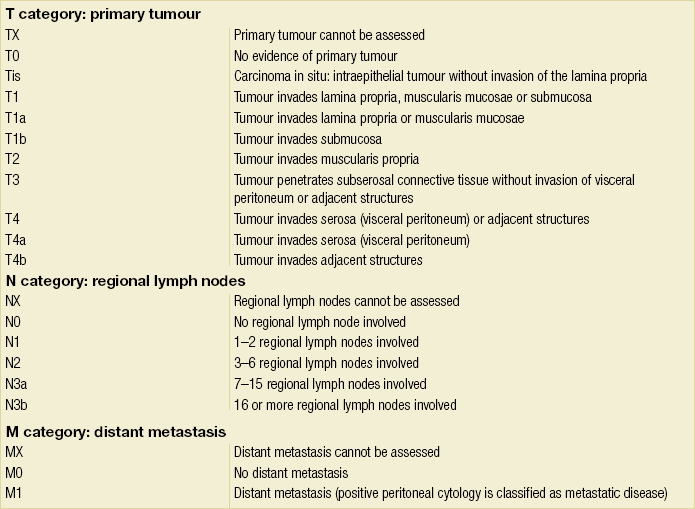
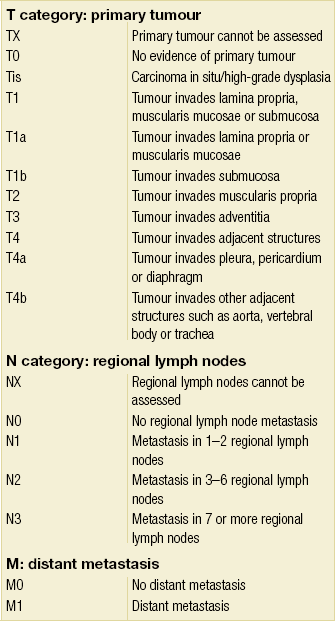
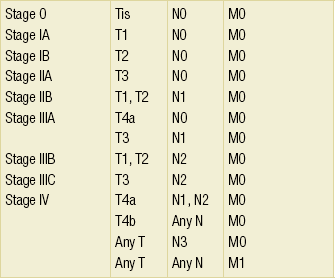
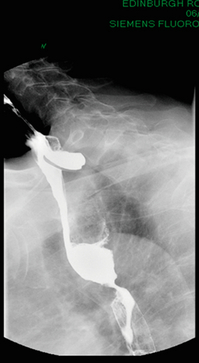
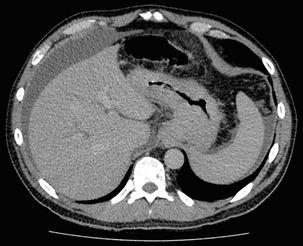
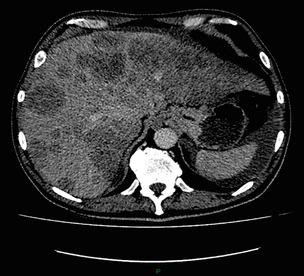
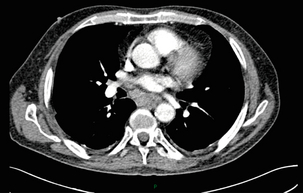
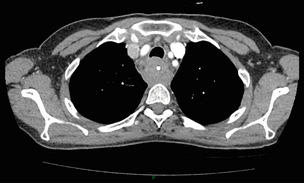
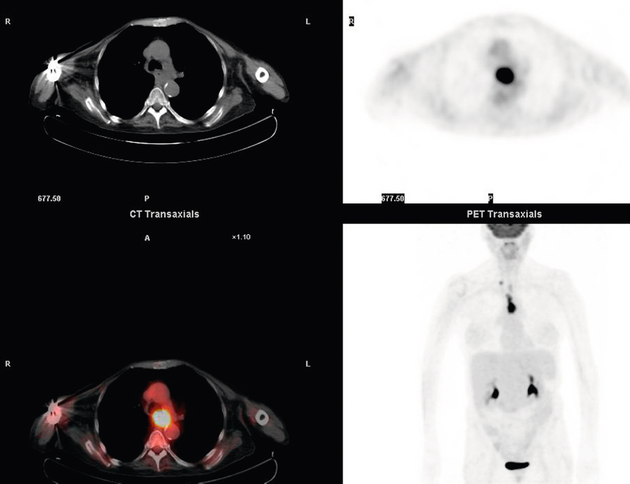
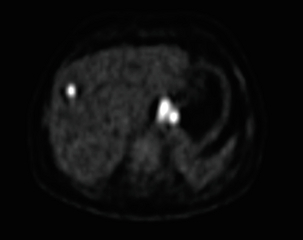
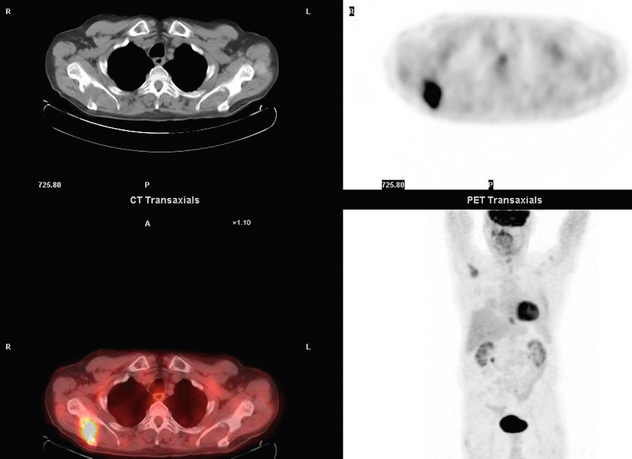
3 A review of this chapter from previous editions highlights the huge changes that have, and continue, to occur in the staging process of oesophageal and gastric cancer. The two diseases now have quite distinct staging protocols with an overlap in the often complex area of junctional tumours. The final aim for each patient must be to reach the best treatment option available with the recognition that for the majority of patients this will be a non-curative decision. The extent of change occurring in the last 20 years is clearly demonstrated if we compare the staging investigations employed in the two large MRC trials of neoadjuvant chemotherapy for oesophageal cancer patients undertaken in the UK.1,2 Assessments before treatment in the OEO2 trial (recruitment from 1992 to 1998) included chest radiography, bronchoscopy for upper third and middle third tumours, and ultrasonography or computed tomography (CT) of the liver. In comparison, for entry into the OEO5 trial (recruitment from 2005 to 2011), staging investigations were spiral/multi-slice CT of chest and abdomen (neck and pelvis when indicated) with oral contrast or water, endoscopic ultrasonography (EUS), laparoscopy where clinically indicated with further options of bone scan, positron emission tomography (PET), laparoscopic ultrasonography and intraperitoneal cytology. Although patients rarely require all of these staging modalities, they do all require to be discussed in this chapter with the indications, benefits and limitations outlined for each. The TNM (‘tumour–node–metastasis’) staging system was devised by Pierre Denoix between 1943 and 1952, where T represents the extent of the primary tumour, N the absence or presence and extent of regional lymph node involvement, and M the absence or presence of distant metastases.3 A single TNM staging classification was agreed between the American Joint Committee on Cancer (AJCC), the Japanese Joint Committee (JJC) and the International Union Against Cancer (UICC) in 1987.4 This classification system has been revised on several occasions with the latest version, TNM7, coming into effect in January 2010.5 It is now routinely used by the majority of centres in Europe and North America and its use is strongly recommended. The development of a staging system for gastric cancer with a worldwide application is difficult as the location of the tumour within the stomach influences survival.6 Distal gastric cancers carry a more favourable prognosis and are more common in Asian populations, with more proximal lesions seen more frequently in western countries. Two thirds of gastric cancers occur in developing countries and the inclusion of molecular or immunohistochemical features of the tumour as part of the staging system would prevent the majority of gastric cancers worldwide being available for comparison.7,8 It is for this reason they do not form part of the current TNM7 classification. TNM7 included major changes in the classification of gastric carcinomas in comparison to TNM6.4,9 One of the most significant is the use of the oesophageal cancer staging system for junctional tumours or any tumour arising within the proximal 5 cm of the stomach and crossing the oesophagogastric junction. Previously, in TNM6, junctional tumours could be staged according to either the gastric or oesophageal system depending on the judgment of the clinician.9 Within the luminal gastrointestinal (GI) tract the T categories have been amalgamated for tumours occurring anywhere from oesophagus to rectum. The T stage classification for gastric cancer has changed significantly, with subdivision of the T1 category into T1a (tumour invades lamina propria or muscularis mucosae) and T1b (tumour invades submucosa) (Table 3.1). The distinction between these two stages has become increasingly important with the development of endoscopic resectional techniques for early-stage gastric tumours. T3 is now invasion of the subserosa without invasion of the visceral peritoneum (T2b in TNM6). T4a is tumour invasion of the serosa (visceral peritoneum), which was T3 disease in TNM6. T4 disease in TNM6 (invasion into adjacent structures) is T4b in the current classification. The extent of lymph node involvement is the most important independent prognostic factor in gastric cancer.10 In the history of the development of the TNM classification there has been a move from the location of lymph node metastases (less than or greater than 3 cm from the primary tumour in TNM4) to the total number of involved lymph nodes.9,11–14 The latest edition categorises the number of involved lymph nodes into narrower groups with the aim of improving prognostic accuracy. The seventh edition of TNM will result in upstaging of patients in comparison to TNM6, with fewer nodes required for entry into the N2 and N3 groups. There is the potential for the N classification to be underestimated if the number of examined nodes is too small. To determine the minimum number required for a correct classification, 926 patients undergoing curative resection for gastric carcinoma were analysed in a study by Ichikura et al.15 The number of metastatic lymph nodes correlated significantly with the number of examined lymph nodes. In patients with pN0 disease those with five to nine examined nodes had a significantly lower survival rate compared to those with 10–14 examined nodes. Interestingly, patients with 10–14 nodes examined had as good a prognosis as those with 15 or more. On the basis of this finding the authors concluded that the minimum number of lymph nodes examined for a correct pN0 classification can be reduced from 15 to 10. However, this is assuming that the cTNM stage of N0 disease is accurate and, although there are reports of improvement in the accuracy rates of preoperative diagnosis in cases of early-stage gastric cancer, it would always be the author’s recommendation that a D2 gastrectomy is performed whenever possible.16 In the pN1 and pN2 categories, patients with 29 or fewer examined nodes tended towards lower survival rates than patients with 30 or more examined nodes. The authors concluded that for pN1–3 classifications, 20 or more nodes should be examined, and examining 30 or more lymph nodes may be desirable. However, many reports do not reach these numbers and only 31% of gastric resections in a UK-based study included 15 or more lymph nodes for histological analysis.17 The prognostic value of metastatic lymph node ratio (the ratio of the number of metastatic lymph nodes to the number of lymph nodes removed) after curative resection has been reported.18,19 Both studies reported the metastatic lymph node ratio as an independent prognostic factor for survival. Among patients with pN2 by the UICC/TNM6 classification, survival in patients with a metastatic lymph node ratio less than 0.1 was significantly better than in those with a higher metastatic lymph node ratio.19 Maximising the total number of lymph nodes removed at the time of resection could decrease the metastatic lymph node ratio below 0.1, adding further evidence for the need for extended lymphadenectomy. The TNM7 stage groupings for gastric cancer are outlined in Table 3.2. 1. Cervical oesophagus. From the lower border of the cricoid cartilage to the thoracic inlet (suprasternal notch), approximately 18 cm from the upper incisor teeth. i. Upper thoracic portion. From the thoracic inlet to the level of the tracheal bifurcation, approximately 24 cm from the upper incisor teeth. ii. Mid-thoracic portion. The proximal half of the oesophagus between the tracheal bifurcation and the oesophagogastric junction. The lower level is approximately 32 cm from the upper incisor teeth. iii. Lower thoracic portion. The distal half of the oesophagus between the tracheal bifurcation and the oesophagogastric junction. The lower level is approximately 40 cm from the upper incisor teeth. This portion is approximately 8 cm in length and includes the abdominal oesophagus. Regional lymph nodes (N stage) are those in the oesophageal drainage area including coeliac axis nodes and paraoesophageal nodes in the neck, but not supraclavicular nodes. It is recommended that at least six lymph nodes are examined from the lymphadenectomy specimen. If fewer than six lymph nodes are present and all are negative the classification remains N0. The TNM7 categories for oesophageal cancer are shown in Table 3.3. The introduction of a stratified classification of nodal involvement has been welcomed by most surgeons. Prior to the publication of the latest TNM7 classification, evidence existed that survival may be predicted by the number of involved lymph nodes.20–22 A review of 336 patients undergoing resection of previously untreated adenocarcinoma and squamous cell carcinoma of the oesophagus and gastro-oesophageal junction reported that patients with more than four involved lymph nodes had survival similar to that of patients with M1 disease.20 Patients with no involved lymph nodes had the best prognosis. The authors identified 18 lymph nodes as the minimal number required for accurate staging. In a multinational, retrospective review of 1053 patients with oesophageal cancer treated with resection alone, recurrent disease had occurred in 40% at 5 years.21 The frequency of systemic disease after oesophagectomy was 16% for those without nodal involvement and progressively increased to 93% in patients with eight or more involved lymph nodes. A recent UK study of oesophageal and junctional adenocarcinomas used a revised node (N) classification based on number of involved lymph nodes (N0, none; N1, one to five; N2, six or more) and location in relation to the diaphragm.22 This demonstrated that a poorer prognosis was associated with increasing nodal involvement and involvement above and below the diaphragm. The TNM7 stage groupings for oesophageal cancer are outlined in Table 3.4. Over time staging investigations have become more numerous and complex, and treatment options more varied. In order to keep abreast of current evidence and to ensure the highest level of expertise is afforded to all patients it is essential that all cancer patients are discussed at a multidisciplinary team (MDT) meeting. This meeting should include surgeons, gastroenterologists, radiologists, radiation and medical oncologists, pathologists, cancer nurse specialists and palliative care physicians. The improved accuracy of CT staging with the involvement of specialist radiologists within the MDT setting has been reported.23 It has also been shown that involvement of the MDT improves overall clinical staging accuracy and is associated with improved outcomes after surgery for gastro-oesophageal cancer.24–26 In a recent review on the introduction of an MDT for patients with oesophageal cancer, there was a significant increase in the percentage of patients receiving complete staging, a multidisciplinary evaluation and adherence to nationally accepted care guidelines.27 The time from diagnosis to treatment significantly decreased, reducing from a mean of 27 to 16 days (P < 0.0001). Dutch guidelines similarly recommend discussion of patients with upper GI malignancies by an MDT. A recent study found that in over one-third of cases the diagnostic work-up or treatment plan proposed by the referring physician was altered after evaluation by the MDT.28 Contrast radiography must be mentioned but cannot be regarded as either a first-line or routine investigation in patients with suspected upper GI malignancy. While there is evidence that double-contrast radiology can diagnose oesophageal and junctional tumours with a sensitivity of 96%, it cannot provide the essential histological confirmation that is obtained at endoscopy and is required for staging purposes.29 It must, however, be stated that in patients with dysphagia with a normal contrast study there is minimal chance that endoscopy will detect any missed oesophageal carcinomas.30,31 If for whatever reason an endoscopy is impossible or not tolerated, a contrast examination may be useful (Fig. 3.1). Flexible upper GI endoscopy is the most important investigation in the diagnosis of oesophageal and gastric carcinoma. In experienced hands it is a safe procedure, with a large UK-based audit of 14 149 procedures reporting a perforation rate of 0.05% and an overall mortality rate of 0.008% during diagnostic endoscopy.32 After cardiopulmonary complications, perforation is the second most important complication. Although historically the majority of endoscopies were performed under sedation, there is an ever increasing number being performed without sedation or under topical oropharyngeal anaesthesia with 100 mg lignocaine spray.33–35 Endoscopy provides accurate information on the location and extent of the lesion and its relationship to anatomical landmarks. Crucially, it also provides the opportunity to obtain a tissue diagnosis with the acquisition of biopsies. It has been shown in patients with carcinoma of the oesophagus that two endoscopic biopsies will provide a positive diagnosis in 95.8% of cases, four biopsies in 97.9% and six biopsies in 100% of cases.36 It is recommended that at least six to eight biopsies are taken at the time of endoscopy to improve the chances of reaching a definitive tissue diagnosis. Not all tumours are negotiable at the time of endoscopy and although dilatation can be performed, the risk of perforation is significantly increased with the risk of rendering a potentially operable tumour inoperable or greatly impairing the prognosis.37 It is therefore advisable when a tumour is stenotic to first obtain an urgent tissue diagnosis before any consideration is given to dilatation. Once a cancer diagnosis has been made, CT is recommended as the initial imaging investigation for both oesophageal and gastric lesions. This allows detection of nodal involvement and metastatic disease and is the most cost-effective investigation.38 Recent progress in multi-detector row CT (MDCT) allows a thinner section thickness in a single breath hold to be obtained, with subsequent improvement in image quality. Patients with gastric cancer require CT of chest, abdomen and pelvis. Scans are performed with intravenous contrast and oral ingestion of either effervescent granules or 1 litre of water to create gastric distention.39,40 The CT appearances of gastric carcinoma are variable and can present with either focal or diffuse wall thickening (Fig. 3.2). Lesions may project into the lumen of the stomach or ulcerate into the wall. With MDCT the overall accuracy in determining the T stage is now in the region of 77–89%.40–46 The ability of CT to detect organ invasion by the primary tumour remains disappointing even with modern scanners. A study from Japan that assessed high-resolution CT and adjacent organ invasion showed that the finding of an absence of fat plane or an irregularity of the border between the tumour and the adjacent organ was not significantly related to invasion.47 However, when the mean densities at the region of interest were measured they were found to be significantly greater at invasion sites than at non-invasion sites. Although this allowed invasion of the pancreas, liver and colon to be assessed with an accuracy of 75%, 61% and 78%, respectively, these authors still found that CT had limited value in differentiating inflammatory adhesions with fibrosis or oedema from true invasion. A recent study reported a marked improvement in T-stage accuracy using a new CT vessel probe reconstruction protocol using a 16-row MDCT.48 When compared with standard axial images the overall accuracy rates for T stage improved from 68% to 94% when compared with final histology. Virtual upper GI endoscopy is a minimally invasive test that utilises three-dimensional (3-D) CT to simulate conventional upper endoscopy images. Images are obtained using both oral and intravenous contrast. The detection rate of gastric lesions using virtual GI endoscopy has been reported to be between 78.7% and 96.7% in early gastric cancer and between 90% and 100% in advanced gastric cancer.49,50 The overall accuracy, sensitivity and specificity for 3-D multi-detector row CT in the preoperative determination of depth of invasion of gastric cancer (T stage) have been reported to be 83.3%, 69.1% and 94.4%, respectively.50 Conventional upper GI endoscopy provides direct visualisation of the mucosa, permits evaluation of colour changes that may be indicative of pathology, and suspicious lesions can be biopsied and the tissue sample evaluated histologically. While virtual upper GI endoscopy using CT is a promising method for the detection and evaluation of upper GI lesions, randomised controlled studies comparing it to conventional upper GI endoscopy are needed to determine its clinical value. Accuracies ranging between 63% and 80% have been reported for N staging by CT when compared with histological staging of the resected specimen (sensitivity 74%, specificity 65%).42–45,51,52 Limitations to CT nodal staging relate to the detection of involved perigastric nodes close to the primary tumour (Fig. 3.3). These lymph nodes often appear confluent with the primary tumour and therefore CT will continue to lack accuracy in nodal staging of some gastric cancers. Figure 3.3 CT image of patient with distal gastric cancer, gastric outlet obstruction and food residue within stomach. One study that retrospectively reviewed the histology of more than 23 000 lymph nodes from gastric cancer resections demonstrated that the mean diameter of a metastatic node was 7.8 mm, and if 5 mm was used as a cut-off, 38% of metastatic nodes would still be missed.53 Improved image quality associated with modern scanners allows the identification of even smaller regional lymph nodes, but the pathological significance of these smaller lymph nodes remains unknown. A recent study reported an accuracy rate of 93% in the identification of para-aortic lymph node metastases from gastric cancer using MDCT.54 Thirteen of 92 (14%) patients undergoing potentially curative resection had para-aortic lymph node involvement on histological examination. Eleven of these were correctly staged preoperatively using MDCT, a sensitivity of 85%. The accuracy, sensitivity and specificity of 3-D multi-detector row CT for lymph node staging were reported to be 75%, 57.4% and 89.3%, respectively.51 CT is useful in the detection of distant metastases and accuracy figures are similar to those seen for oesophageal malignancy (Fig. 3.4). CT, however, is limited in its ability to detect transcoelomic spread and the presence of peritoneal seedlings. In a study of 78 patients listed for curative gastrectomy for gastric cancer based on CT findings, 23 (29.5%) had undetected peritoneal spread at the time of laparoscopy.55 It is recommended that all patients being considered for curative gastrectomy undergo a staging laparoscopy with peritoneal washings. The presence of CT-defined minimal ascites (< 50 mL) has been reported not to affect survival on a stage-for-stage basis in patients with gastric cancer without peritoneal metastases.56 Of those with CT-defined minimal ascites, 28.1% had peritoneal metastases confirmed at surgery. Further improvements in the accuracy of CT staging may be achieved through establishment of radiologists with a special interest. One report demonstrated improved levels of sensitivity and specificity among radiologists who regularly stage patients with gastric cancer, with an associated reduction in the open-and-close laparotomy rate.57 Such findings provide additional support for the formation of specialist multidisciplinary teams for the management of gastro-oesophageal cancer. Conventional CT has historically diagnosed T4 lesions with high accuracy rates. This is because the criteria for staging T4 lesions are based on obliteration of the fat layer or the angle between the tumour and the adjacent organs (Fig. 3.5).58–60 Oesophageal wall thickness has been used for earlier T stages because tumour cannot be sufficiently differentiated from normal oesophageal wall.61,62 Accuracy rates for T-stage detection using spiral CT when compared with histopathological stage of resected specimens have been reported as between 43% and 92%.63–65 Wu et al. reported the accuracies of T staging using the following criteria: T1 and T2, oesophageal wall thickness < 5 mm; T3, oesophageal wall thickness > 5 mm; and T4, invasion into adjacent organs.66 In their study, the accuracy values of the respective T staging were 75% for T1/T2, 79% for T3 and 64% for T4. According to these criteria T1 lesions cannot be differentiated from either T2 lesions or from normal oesophageal wall. A recent study from Japan reported improved accuracy rates for T staging of early oesophageal carcinomas (T1a and T1b).67 A dual-phase (arterial and venous phase) contrast-enhanced CT protocol with MDCT was used. All lesions classified as T1 lesions were T1b with no T1a lesions visualised. This differentiation is important as T1a lesions can be considered for endoscopic mucosal resection, with T1b requiring more radical treatment. In addition, the nodal involvement rate increases from 1.3% in T1a lesions to 22% in T1b lesions.68 Figure 3.5 CT image of patient with squamous cell carcinoma (SCC) of oesophagus from 20 to 27 cm with T4 invasion into trachea. A fine-bore feeding tube is in situ. Accuracy for N-stage disease ranges between 27% and 86% (sensitivity 48–68% with a specificity of 90–95%) (Fig. 3.6).63–65,69,70 The difficulty in the accurate identification of involved lymph nodes is the reliance on size to differentiate between malignant and benign pathology. The size of the lymph node that different authors regard as a criterion for malignant involvement varies from 5 to 15 mm.71 Lymph nodes of more than 1 cm in diameter can, however, be seen within the mediastinum in healthy people, particularly those with coexisting chest problems, and nodes of normal size may contain metastatic deposits.72 CT and EUS are complementary techniques in staging oesophageal cancer patients.73,74 A comparison of EUS and CT identification of involved lymph node stations in 121 patients with squamous cell carcinoma of the oesophagus reported an overall accuracy of 64% for EUS (sensitivity 68%, specificity 58%, positive predictive value (PPV) 68%), 51% for CT (sensitivity 33%, specificity 75%, PPV 64%), and 64% for CT and EUS in combination (sensitivity 74%, specificity 50%, PPV 66%).74 However, some metastatic lymph nodes in the neck and abdomen are only detectable by CT, and it was recommended that both EUS and CT should be undertaken for routine examination prior to treatment of oesophageal cancer. The role of fluorodeoxyglucose (FDG)-PET in gastric cancer is not as well established as in oesophageal cancer. Reported detection rates of primary tumours vary between 60% and 90% and depend on the histopathological characteristics of the primary tumour.75,76 Stahl et al. reported on a series of 40 gastric cancer patients and found that tumours with a non-intestinal growth type according to the Lauren classification showed significantly lower FDG uptake than tumours of the intestinal growth type.77 Non-mucinous carcinomas accumulated significantly more FDG than mucinous ones. This finding has also been reported by Yamada et al., who found a higher standardised uptake value (SUV) in the tubular adenocarcinoma group than in the mucinous and signet-ring cell adenocarcinoma group.78 These findings have been confirmed in a more recent study that also showed that the expression of the glucose transporter (GLUT-1) significantly correlated with the maximum calculated SUV: 76% of signet-ring cell carcinomas did not show GLUT-1 expression.79 Conflict still exists on the role of PET in the management of patients with oesophageal cancer, but it has been widely adopted in most large centres. The Scottish Intercollegiate Guidelines Network (SIGN) previously concluded that PET is not routinely indicated in staging gastro-oesophageal tumours.80 To date, no randomised trial has been undertaken to determine the role of FDG-PET or PET/CT in staging upper GI cancer. Such a trial is unlikely to occur as it is now an established staging tool in upper GI cancer despite the lack of published evidence. In 2009 the Scottish National PET Advisory Group recommended the routine use of PET/CT in staging patients with potentially operable oesophageal cancer. Changes in management with the addition of FDG-PET to the staging protocol in patients with oesophageal cancer are reported to occur in anything from 3% to 41% of patients.81–86 This huge variation may be related to the point in the staging pathway at which PET has been introduced. In a study of 199 patients, FDG-PET was performed only after a full preoperative staging protocol with MDCT, EUS and external ultrasonography of the neck, both combined with selective fine-needle aspiration cytology.86 Only patients considered eligible for curative surgery after these investigations underwent FDG-PET. FDG-PET revealed suspicious hot spots in 15.1% of patients but metastases were confirmed in only 4.0%. All upstaged patients had clinical stage III–IV disease before FDG-PET. In 3.5% the hot spots appeared to be synchronous neoplasms, mainly colonic polyps. The remaining 7.5% were false positive, leading to unnecessary additional investigations. The authors concluded that the diagnostic benefit of the addition of PET is limited after state-of-the-art staging, and so broad implementation in daily clinical practice is questionable. However, it does not appear sensible on a cost basis to introduce PET at the end of the staging pathway. At the time of writing the costs of MDCT in the UK are approximately £500, PET/CT £1000 and EUS £1800. It would therefore seem reasonable to perform the investigations in this order, reserving the most expensive and invasive tests until the end of the pathway. PET scans have a limited role in evaluating the T stage of a tumour due to their limited spatial resolution of approximately 6 mm (Fig. 3.7). In a comparison of CT, PET and EUS in the initial staging of patients with oesophageal cancer, Lowe et al. reported correct T staging by CT and PET in only 42% of patients compared with 71% with EUS.87 Superficial and in-situ malignancies of the oesophagus can be difficult to detect with PET, with one study reporting 100% of tumours confined to the mucosa (Tis and T1a) being FDG negative.88 Kato et al. reported that just 18% of T1a tumours and 61% of T1b tumours were FDG-PET positive.89 Low FDG uptake has also been reported in tumours with undifferentiated or mucinous features.90,91 Possible explanations for this include differences in the glucose transporter mechanism, reduced intracellular hexokinase activity resulting in a low rate of FDG phosphorylation, a low volume of metabolically active tumour cells or differences in tumour vascularity. Figure 3.7 FDG-PET/CT image demonstrating increased uptake in a mid-oesophageal SCC. Increased uptake is evident in a right supraclavicular lymph node. Early studies of FDG-PET in staging oesophageal cancer highlighted the difficulty in differentiating nodal disease adjacent to the primary tumour from the primary tumour itself.92–94 In a review of 12 published studies, pooled sensitivity and specificity for the detection of locoregional metastases were 0.51 (95% confidence interval (CI) 0.34–0.69) and 0.84 (95% CI 0.76–0.91), respectively.95 For distant metastases, pooled sensitivity and specificity were 0.67 (95% CI 0.58–0.76) and 0.97 (95% CI 0.90–1.0), respectively. A more recent meta-analysis reported similar results, where pooled sensitivity and specificity of FDG-PET for regional lymph node metastases were 0.57 (95% CI 0.43–0.70) and 0.85 (95% CI 0.76–0.95), respectively.96 The introduction of integrated CT and PET has improved the accuracy of staging for patients with oesophageal cancer.97,98 In a study of 45 patients with thoracic oesophageal squamous cell cancer, PET/CT was superior to PET alone in the detection of locoregional nodal involvement.98 Sensitivity, specificity and accuracy of PET/CT were 94%, 92% and 92%, respectively, whereas PET alone was 82% sensitive, 87% specific and 86% accurate. The main role of PET/CT is the identification of distant metastases not evident on CT (Figs 3.8 and 3.9). This prevents unnecessary surgery in patients with incurable disease and allows for appropriate palliative treatments to be offered. In two similar sized meta-analyses, each of over 400 patients, the reported sensitivities and specificities for PET in the detection of distant metastases were similar.95,96 Twelve studies were analysed in the study by van Westreenen et al., with a pooled sensitivity of 67% (95% CI 58–76%) and a specificity of 97% (95% CI 90–100).95 In the report by Van Vliet et al., nine studies were analysed, with a pooled sensitivity of 71% (95% CI 62–79%) and a specificity of 93% (95% CI 89–97).96 Interestingly, only three studies appeared in both meta-analyses. Figure 3.8 FDG-PET/CT image demonstrating increased uptake in abdominal lymph nodes and a solitary metastasis in the right lobe of the liver. The primary lesion was a distal oesophageal adenocarcinoma. Figure 3.9 FDG-PET/CT image demonstrating a bone metastasis in right scapula from a distal oesophageal adenocarcinoma. The bone lesion was not detected on original review of the CT images. Accepting that the main role of PET is the detection of metastatic disease, it is debatable whether the routine use of PET is justified in patients with early lesions that are often not visualised on PET and have a low risk of nodal involvement let alone metastatic disease.68,88,89 It is possible that patients with no obvious lymph node involvement on CT gain little from PET but require EUS for confirmation of N0 status and if confirmed should be offered immediate surgery. Those patients with locoregional lymphadenopathy on CT are more likely to have undetected metastases and should be offered PET/CT.
Staging of oesophageal and gastric cancer
Introduction
Staging classifications
Gastric cancer staging
Oesophageal cancer staging
Multidisciplinary team
Staging investigations
Contrast radiography
Endoscopy
Computed tomography (CT)
Gastric cancer
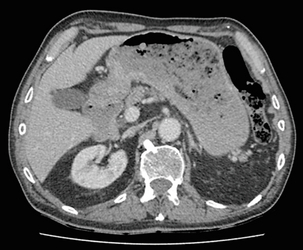
Oesophageal cancer
Positron emission tomography (PET)
Oesophageal cancer
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Staging of oesophageal and gastric cancer