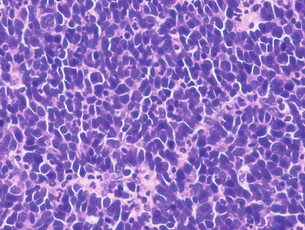
1
Pathology of oesophageal and gastric tumours
Oesophagus
Epithelial tumours of the oesophagus and the gastro-oesophageal junction
Malignant tumours
Squamous cell carcinoma: Squamous cell carcinoma is the most common malignant tumour of the oesophagus worldwide and affects men two to ten times more often than females, with an average age between 50 and 60 years at time of diagnosis. There is a marked geographic and ethnic variation in incidence. Incidence rates are highest in Iran, China, South America and Eastern Africa and are higher in African-Americans than Caucasian-Americans regardless of gender.
The intake of hot beverages has been shown to increase the risk of squamous cell carcinoma. Furthermore, dietary factors such as a lack of fresh fruit and vegetables and high intake of barbecued meat or pickled vegetables most likely play a role in the aetiology of squamous cell carcinoma. Human papilloma virus infection has been implicated in the pathogenesis of oesophageal squamous cell carcinoma, but its precise role is still controversial at present. Patients with achalasia have an increased risk of developing cancer compared to the normal population.4 The risk of developing oesophageal carcinoma is also increased in patients with coeliac disease,5 Plummer–Vinson syndrome (also called Paterson–Kelly syndrome),6 tylosis (also called focal non-epidermolytic palmoplantar keratoderma),7,8 previous ingestion of corrosive substances,9 Zenker’s diverticulum10 or after ionising radiation.11 In the Asian population, polymorphisms in ALDH1B1 and ALDH2, both genes encoding aldehyde dehydrogenases, are associated with squamous cell carcinoma.12
Oesophageal squamous cell carcinomas are found in the upper, middle and lower third of the oesophagus in a ratio of approximately 1:5:2. The macroscopic appearance depends on the depth of tumour invasion and is classified into four different types according to the Japanese classification for oesophageal cancer,13 which is similar to the macroscopic classification of gastric cancer (see Fig. 1.8 below). Approximately 60% of squamous cell carcinomas show an exophytic or fungating growth pattern, 25% are ulcerative and 15% are infiltrative (Fig. 1.1). However, the macroscopic appearance of all cancers can significantly change as a result of neoadjuvant chemotherapy or chemoradiation, with tumour shrinkage, extensive necrosis and fibrosis.
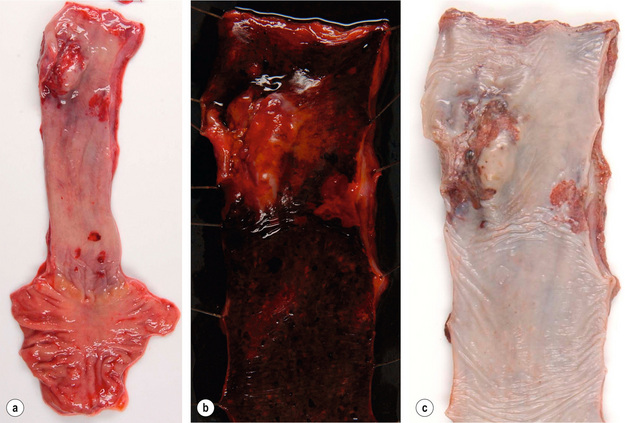
Figure 1.1 Oesophageal squamous cell carcinoma located in the middle oesophagus. (a) Fresh oesophagectomy specimen with a polypoid exophytic tumour growth and a smaller flat (red coloured) mucosal abnormality. (b) Lack of (dark) iodine staining in the abnormal areas. (c) Same specimen after fixation. Courtesy of Dr Tomio Arai, Tokyo.
Squamous cell carcinomas invade both horizontally and vertically. In the West, 60% of patients have carcinomas that have invaded beyond the muscularis propria and have regional lymph node metastases at the time of diagnosis, whereas in Japan up to 40% of all resected carcinomas are superficial or early carcinomas involving mucosa and submucosa only.14 The frequency of lymph node metastases is related to the depth of tumour invasion and has been reported as less than 5% for intramucosal carcinomas and up to 45% for submucosal carcinomas. Although tumours located in the upper third of the oesophagus are more likely to spread to cervical and upper mediastinal nodes, a significant proportion will also spread to perigastric nodes.
Distant metastases due to haematogenous spread are most commonly found in liver, lung, adrenal gland and kidney.18
Histologically, squamous cell carcinomas are characterised by keratinocyte-like cells that may or may not have intercellular bridges and show a variable degree of keratinisation (Fig. 1.2). Depending on the extent of mitotic activity, nuclear atypia and degree of squamous differentiation including degree of keratinisation, squamous cell carcinomas are graded as well, moderately or poorly differentiated.12 The histology of squamous cell carcinoma can change dramatically after neoadjuvant chemo(radio)therapy and then typically shows extensive necrosis, inflammation, fibrosis and foreign body-type granulomas around keratin pearls. There is currently no consensus on how to grade tumour regression. The regression grading according to Mandard et al.19 considers the relative proportion of residual viable tumour cells and fibrosis in the primary cancer and is probably currently the one most commonly used in the UK. Very recently, a grading system to assess tumour regression in lymph nodes has been proposed and showed prognostic significance in a small series of patients.20
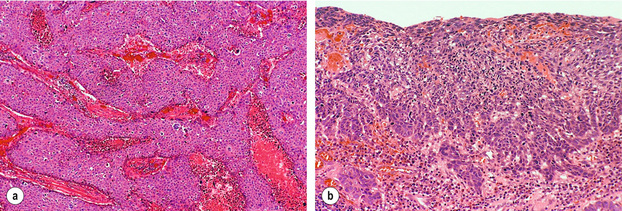
Figure 1.2 Histological images from specimen in Fig. 1.1. (a) Histology of the nodule shows poorly differentiated squamous cell carcinoma with no evidence of keratin formation and necrosis (pink material) between strands of neoplastic cells. (b) Histology of the flat lesion shows early infiltrative squamous cell carcinoma. Courtesy of Dr Tomio Arai, Tokyo.
Three main variants of squamous cell carcinoma have been described:12
1. Verrucous carcinoma of the oesophagus is a rare, locally aggressive tumour that is more common in males. Macroscopically, the tumour has an exophytic papillary appearance and tumours are usually very large before they become clinically apparent. Microscopically, the tumour is very well differentiated with minimal atypia. Superficial biopsies are often insufficient to distinguish between a squamous papilloma, pseudoepitheliomatous hyperplasia and verrucous carcinoma.21
2. Spindle cell carcinoma (also known as carcinosarcoma, sarcomatoid carcinoma and polypoid carcinoma) is a polypoid tumour located in the middle or lower third of the oesophagus. Histologically, the tumour is a mixture of a well-differentiated squamous cell carcinoma and a high-grade spindle cell component that can show osseous, cartilaginous or skeletal muscle differentiation.22 Spindle cell carcinomas are highly aggressive carcinomas, with 5-year survival rates of 10–15%.23
3. Basaloid squamous cell carcinoma is an unusual variant of squamous cell carcinoma that needs to be distinguished from ‘pure’ squamous cell carcinoma, adenoid cystic carcinoma and neuroendocrine tumours. It is a highly aggressive carcinoma with a very poor prognosis. Histologically, this tumour shows the characteristic basaloid cells together with a mucoid hyaline-like substance, as well as multiple other components.
Precursor lesions of squamous cell carcinoma: Oesophageal squamous cell carcinoma development is believed to be a multistep process from normal squamous epithelium via intraepithelial neoplasia (synonym: dysplasia) to invasive carcinoma based on findings in high-risk populations where dysplasia predates the development of carcinoma by approximately 5 years.24,25 In general, dysplasia is defined as the presence of unequivocal neoplastic cells within the epithelium. Squamous cell dysplasia is classified as ‘low grade’ when architectural and cytological abnormalities are seen in the basal half of the squamous epithelium with preserved maturation of the upper half, and as ‘high grade’ when more than the bottom half shows architectural and cytological abnormalities. Full-thickness dysplasia of the squamous epithelium is referred to as ‘carcinoma in situ’ by some authors.
Molecular pathology of squamous cell carcinoma: Up to 80% of squamous cell carcinomas show mutation with consecutive loss or inactivation of the tumour suppressor gene p53 (the ‘guardian’ of the genome located on the short arm of chromosome 17), of the retinoblastoma gene RB and of p16.26 Amplification (e.g. an increase in gene copy number) and subsequent protein overexpression of cyclin D1, a cell cycle regulating gene, occurs in 20–40% of squamous cell carcinomas. Inactivation of FHIT (fragile histidine triad gene, a presumed tumour suppressor gene on chromosome 3p14), DLEC1 (deleted in lung and oesophageal cancer-1) and DEC1 (deleted in oesophageal cancer-1) by genetic or epigenetic mechanisms promoting cancer cell growth has recently been shown. Amplifications of several proto-oncogenes and growth factors such as FGF4 and FGF6 (fibroblast growth factors 4 and 6), EGFR (epidermal growth factor receptor) and MYC have also been found in oesophageal squamous cell carcinoma. Some of these changes, such as p53 mutations, appear to be an early event as they have also been demonstrated in squamous cell dysplasia. The presence of such genetic changes may be used to select patients for targeted therapy with antibodies or small-molecule inhibitors in the near future.
Adenocarcinoma: Population-based studies in the USA and Europe indicate that the incidence of oesophageal adenocarcinoma, adenocarcinoma of the gastro-oesophageal junction and proximal stomach has doubled between the 1970s and late 1980s, and continues to increase by 5% every year.2,27 Countries with the highest incidence of oesophageal adenocarcinoma are the UK, Australia, the Netherlands and the USA. Oesophageal adenocarcinoma is much more common in males (male:female ratio 4:1 to 7:1) and 80% of oesophageal adenocarcinomas occur in the white population.
The relative risk of developing adenocarcinoma in patients with Barrett’s oesophagus is of the order of 30–60,27 but only 5% of patients with oesophageal adenocarcinoma have had a previous diagnosis of Barrett’s oesophagus.28 Other risk factors of oesophageal adenocarcinoma are tobacco smoking, obesity (which may promote gastro-oesophageal reflux), and use of medications that relax the gastro-oesophageal sphincter. No clear association has been found between alcohol consumption or diet and adenocarcinoma. Case–control studies seem to indicate that infection with Helicobacter pylori is protective against oesophageal adenocarcinoma.
There is an ongoing debate whether adenocarcinoma in the proximity of the oesophagogastric junction should be classified as oesophageal or gastric carcinoma. This is mainly related to the fact that there is no consensus on the definition of the ‘gastro-oesophageal junction’ and ten different definitions are listed in the fourth edition of the WHO classification of digestive cancer 2010.12 Siewert and Stein29 defined adenocarcinoma of the gastro-oesophageal junction as ‘tumours that have their centre within 5 cm proximal and distal of the anatomical cardia’ and suggested three tumour types based on the anatomical location of the tumour centre determined by a combination of radiography, endoscopy, computed tomography and intraoperative appearance:
• Type I. Adenocarcinoma of the distal oesophagus, which usually arises from an area with specialised intestinal metaplasia (i.e. Barrett’s oesophagus) and which may infiltrate the gastro-oesophageal junction from above. This entity is also referred to as ‘Barrett carcinoma’. These adenocarcinomas have their centre within 1–5 cm above the cardia.
• Type II. True carcinoma of the cardia arising from the gastric cardia epithelium or from short segments with intestinal metaplasia at the gastro-oesophageal junction. This entity is also referred to as ‘junctional carcinoma’. These adenocarcinomas have their centre within 1 cm above and 2 cm below the cardia.
• Type III. Subcardial gastric carcinoma that infiltrates the oesophagogastric junction and distal oesophagus from below. This entity is also referred to as ‘proximal gastric carcinoma’. These adenocarcinomas have their centre within 2–5 cm below the cardia.
Adenocarcinoma associated with Barrett’s oesophagus: Columnar epithelium in the oesophagus in combination with ulceration and oesophagitis was first described by Norman Barrett in 1950, who was convinced that this was due to a congenitally short oesophagus.30 Moersch et al.31 and Hayward32 were the first to suggest that the columnar lining of the oesophagus might be an acquired condition due to gastro-oesophageal reflux. Experiments conducted by Bremner et al.33 in 1970 in a dog model of gastro-oesophageal reflux strongly supported this concept.
The risk of developing adenocarcinoma appears to be related to the length of the metaplastic mucosa, with 3 cm being used as the cut-off between a ‘short’ and a ‘long’ segment Barrett’s oesophagus. Further details of Barrett’s oesophagus including the proposed metaplasia–dysplasia–adenocarcinoma sequence can be found in Chapter 15.
Barrett’s associated adenocarcinomas are located almost exclusively in the distal third of the oesophagus and often infiltrate into the proximal stomach (Fig. 1.3). Up to 50% of adenocarcinomas show a macroscopic infiltrative growth pattern and only 5–10% are polypoid. Histologically, oesophageal adenocarcinomas are typically papillary and/or tubular (intestinal type according to the Laurén classification35) and are graded as well, moderately or poorly differentiated according to the proportion of tumour that is composed of glands.12 Approximately 10% of all oesophageal adenocarcinomas are of mucinous or signet-ring cell type. Most patients present with locally advanced disease, where the adenocarcinoma has infiltrated beyond the deep muscle layer into the perioesophageal tissue and involves regional lymph nodes in up to 75% of cases. Should the patient present with early disease, it is important to remember that there is a double muscularis mucosae in almost all cases with Barrett’s oesophagus. Carcinomas infiltrating between the two layers of the muscularis mucosae are still to be classified as ‘intramucosal’ pT1a cancers. However, carcinomas that have infiltrated into this double muscularis mucosae layer may be associated with a higher frequency of lymphoangioinvasion and lymph node metastases.36 This has implications for endoscopic treatments.
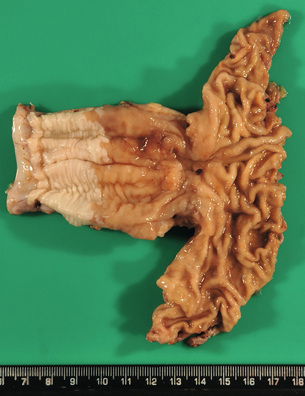
Figure 1.3 Barrett’s oesophagus with adenocarcinoma is seen on the left. An irregular, partly ulcerated tumour is located at the gastro-oesophageal junction. Between the proximal edge of the tumour and the squamous lined oesophagus is metaplastic columnar epithelium. The squamocolumnar junction (border between the pale appearing squamous epithelium and brownish-appearing metaplastic epithelium) is located at least 2.5 cm proximal to the gastro-oesophageal junction. Courtesy of Dr B. Disep, Newcastle.
Variants of oesophageal adenocarcinoma:
1. Truly non-Barrett’s oesophagus-associated adenocarcinoma of the oesophagus is rare and arises either from heterotopic gastric mucosa (so called ‘gastric inlet’), which can be anywhere in the oesophagus or from the epithelium of submucosal oesophageal glands.
2. Adenoid cystic carcinoma is also very rare. These carcinomas are histologically identical to salivary gland-type adenoid cystic carcinoma and occur more frequently in females.37 These carcinomas arise from submucosal oesophageal glands. They usually form well-circumscribed solid nodules in the submucosa and the overlying squamous epithelium shows no abnormalities. Most tumours show some differentiation towards squamous, glandular or even small cell elements which could indicate an origin from a multipotential stem cell.
Precursor lesions of oesophageal adenocarcinoma and molecular pathology of oesophageal adenocarcinoma are discussed in more detail in Chapter 15.
Neuroendocrine tumours of the oesophagus: Oesophageal neuroendocrine tumours are very rare, representing less than 8% of all oesophageal carcinomas. The majority of them are poorly differentiated neuroendocrine carcinomas (also known as small cell carcinomas) that are highly aggressive and median survival is usually 6–12 months or less. Macroscopically, they appear as exophytic or ulcerative growths measuring on average 6 cm at presentation. Histologically, these may appear as homogeneous tumours (Fig. 1.4) or as a mixture of squamous and mucoepidermoid elements. The histological features including immunohistochemical markers are similar to small cell carcinoma of the lung and the possibility of metastatic or direct spread from the lung should always be considered in the differential diagnosis.
Mesenchymal tumours of the oesophagus:
Leiomyoma: Leiomyoma is the most common benign mesenchymal tumour of the oesophagus, which is twice as frequent in males as females. Leiomyomas are typically located in the distal or middle oesophagus. Most are less than 3 cm in size and form a hard white-greyish mass. In contrast to gastrointestinal stromal tumours, leiomyomas are immunoreactive for desmin and smooth muscle actin and negative for KIT (CD117) and DOG1.
Granular cell tumour: Granular cell tumours are found in the skin, mouth and throughout the gastrointestinal tract, but most frequently in the oesophagus. Nearly two-thirds of these tumours have been found in the lower third of the oesophagus and arise in the submucosa. The covering squamous epithelium is often thickened and may show pseudoepitheliomatous hyperplasia. The characteristic tumour cells are uniform plump cells with granular cytoplasm that stain with periodic acid–Schiff and S-100 protein.
Stomach
Fundic gland polyps
Fundic gland polyps are the most common type of gastric polyps and were originally described in patients with familial adenomatous polyposis (FAP) syndrome.38 Sporadic fundic gland polyps are found in up to 11% of patients, are more common in middle-aged women and are typically single or few, measuring less than 0.5 cm. The incidence of fundic gland polyps is low in patients with H. pylori infection and high in patients taking proton-pump inhibitors.39 While low-grade dysplasia is frequent in FAP patients with fundic gland polyps, dysplasia is rare in sporadic cases.40
Hyperplastic polyps are composed of epithelial and stromal components, and are most frequently found in the antrum of patients with inflamed or atrophic gastric mucosa. A recent review of more than 8000 gastric polyps showed that only 14% were hyperplastic polyps.39 Hyperplastic gastric polyps are thought to arise as a hyperproliferative response of the gastric foveolae to tissue injury. Removal of the underlying injury such as H. pylori infection resulted in regression of the hyperplastic polyps in 70% of patients.42 1–20% of hyperplastic polyps show foci of dysplasia, p53 mutations, chromosomal aberrations and microsatellite instability, which seem to be related to larger size (> 2 cm).43 Hyperplastic polyps should be regarded as surrogate markers of cancer risk and synchronous or metachronous gastric carcinomas have been reported in up to 6% of cases.
Sporadic intestinal-type adenomas are most common in patients over 50 years of age, three times more frequent in men and most commonly found on the lesser curve of the antrum. They are usually solitary, less than 2 cm in diameter, well circumscribed, pedunculated or sessile, and their prevalence varies widely from 4% in Western countries to 27% in Japan. Adenomatous polyps are precursors of gastric adenocarcinomas and the risk of adenocarcinoma seems to increase with increasing size. Fifty per cent of adenomatous polyps > 2 cm harbour an adenocarcinoma.44
Polyposis syndromes
Hamartomatous polyps in the stomach have been found in patients with Peutz–Jeghers syndrome, juvenile polyposis, Cronkite–Canada syndrome and Cowden disease. With the exception of Peutz–Jeghers polyps, the histological features of these polyps overlap with those of sporadic hyperplastic polyp and the pathological diagnosis of a ‘syndromic polyp’ will require knowledge of the suggestive clinical context. All patients with the above mentioned polyposis syndromes have an increased risk of developing gastric carcinoma that appears to be highest in patients with Peutz–Jeghers syndrome at 30%.45 Up to 80% of patients with Peutz–Jeghers syndrome have a germ-line mutation of the STK11/LKB1 gene that encodes an enzyme responsible for cell division, differentiation and signal transduction. The most common genetic alterations in patients with juvenile polyposis are germline mutation of SMAD4 or BMPR1A, both genes implicated in the transforming growth factor (TGF)-β signalling pathway. Cowden disease is caused by germline mutations of PTEN resulting in multiple hamartomas involving multiple different organs. Cronkite–Canada syndrome is a non-inherited polyposis syndrome of unknown pathogenesis.
Gastric carcinoma
Epidemiology of gastric carcinoma
Despite a steady decline of gastric carcinoma incidence at a rate of approximately 5% per year since the 1950s,46 gastric carcinoma is still the fourth most common carcinoma in the world, with one million people newly diagnosed per year, representing 8% of all new cancers diagnosed per year in the world. Age-standardised incidence rates of gastric carcinoma are twice as high in males as in females and show prominent geographical variation, ranging from 3.9 in Northern Africa to 42.4 in Eastern Asia per 100 000 males.47 Seventy-five per cent of all new gastric carcinoma cases are diagnosed in Asia. Gastric carcinoma is the second leading cause of cancer death in both sexes worldwide, being responsible for 10% of all cancer deaths. A male:female ratio of 2:1 has been reported for non-cardia gastric carcinoma in contrast to a male:female ratio of 5:1 for gastric cardia carcinoma.48
Aetiology and risk factors of gastric carcinoma
Ten per cent of gastric carcinomas show familial clustering, but only 1–3% of gastric carcinomas are related to identified inherited gastric carcinoma predisposition syndromes such as hereditary diffuse gastric carcinoma, hereditary non-polyposis colon cancer (Lynch syndrome), familial adenomatous polyposis, Peutz–Jeghers syndrome, Li–Fraumeni syndrome, and familial breast and ovarian cancer.49,50
One of the defining characteristics of the hereditary diffuse gastric carcinoma syndrome (HDGC) is the presence of a germline CDH1 (E-cadherin) mutation.51 CDH1 mutations have been found in hereditary as well as sporadic diffuse-type gastric carcinomas, but not in intestinal-type gastric carcinomas. CDH1 mutations in sporadic diffuse-type gastric carcinoma cluster in exons 7–9, whereas CDH1 germline mutations are spread over the whole length of the gene in HDGC patients, making genetic testing very time consuming as the whole CDH1 gene might need to be sequenced.52 Patients diagnosed with HDGC have an increased risk of lobular breast cancer and signet-ring colon cancer, and should undergo appropriate surveillance for these diseases.53 The penetrance of the gene varies between 70% and 80%, and the lifetime risk of developing gastric carcinoma in mutation carriers is 67% in men and 83% in women. In order to identify patients that should be offered CDH1 mutation testing, including appropriate genetic counselling, the updated recommendations of the International Gastric Cancer Linkage Consortium (IGCLC) should be followed51 (Box 1.1).
The resection specimen should be worked up and reported according to the recommendations of the IGCLC.51
Helicobacter pylori infection increases the risk of gastric carcinoma up to sixfold and hence represents one of the most important environmental risk factors for the development of gastric carcinoma. Humans are the only known host for H. pylori that can colonise the body and the antrum (Fig. 1.5). The development of gastric carcinoma after H. pylori infection has been considered as a multistep process progressing from chronic active pan- or corpus predominant gastritis to increasing loss of gastric glands (atrophy), replacement of the normal mucosa by intestinal metaplasia and malignant transformation.54–56 Most H. pylori-infected individuals will remain asymptomatic and only 1–5% of the infected population will develop gastric carcinoma, a phenomenon that has been attributed to different bacterial strains, host-inflammatory genetic susceptibility and in particular the H. pylori virulence factors vacuolating cytotoxin antigen (VacA) and cytotoxin-associated gene A antigen (CagA).54,57,58
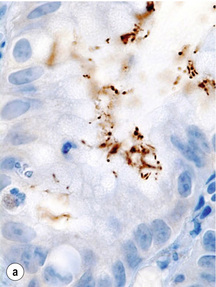
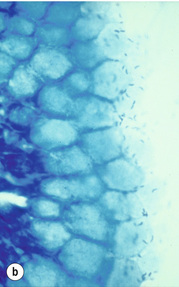
Figure 1.5 Helicobacter pylori. The Gram-negative, spiral-shaped, 2.5 to 5 μm long bacterium can be found on the gastric surface epithelium within the mucous layer. (a) Immunohistochemical staining demonstrates the organisms as brown rods. (b) In the modified Giemsa staining, the organisms appear light blue.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree