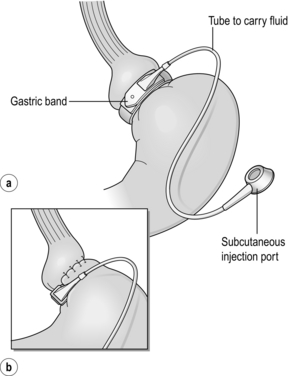
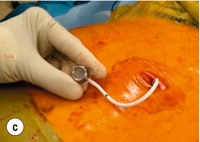
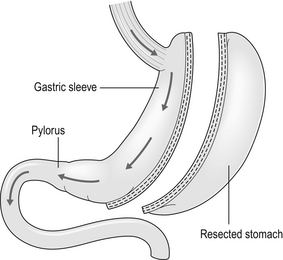
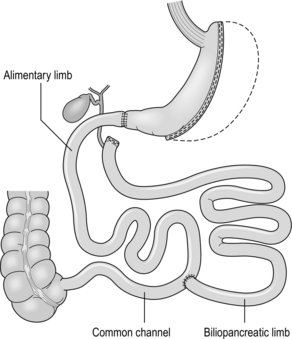
20 Only a few operations have stood the test of time. The original jejuno-ileal bypass was abandoned due to the blind loop syndrome and resulting liver and bone metabolism problems. The vertical banded gastroplasty (VBG) has been superseded by gastric banding due to the advantage of being able to adjust the size of the gastric stoma. The gastric bypass has evolved from a continuously stapled horizontal gastroplasty and loop gastroenterostomy into a smaller pouch with a Roux-en-Y reconstruction.1,2 The Magenstrasse and Mill procedure has evolved into the sleeve gastrectomy, an operation that is rapidly being taken up worldwide due to its perceived ease and lesser risk compared to gastric bypass.3 Other procedures specifically for type 2 diabetes, such as duodeno-jejunal bypass and a temporary implanted endoscopic sheath that prevents absorption in the duodenum and proximal jejunum (‘Endobarrier’), are being evaluated. The revolution of laparoscopic surgery has allowed most bariatric surgery to be performed laparoscopically with low mortality and morbidity. As a result, bariatric surgery is now established as a cost-effective intervention that should be increasingly provided. Obesity is a chronic, relapsing, debilitating, lifelong disease, recognised by the World Health Organisation as a global pandemic.4 The influential Foresight report estimated that by 2010 28% of women and 33% of men in the UK would be obese, rising to 50% of women and 60% of men and, even more worrying, 25% of children as well by 2050.5 The definitions of obesity are shown in Table 20.1. The term ‘obesogenic environment’ was used to describe the ‘results of people responding normally to the obesogenic environments they find themselves in’, given the current trends of reduced physical activity and easily available, highly advertised, relatively cheap, energy-dense foods.6 An example of an obesogenic food environment is the observation that the price per calorie of healthy foods (whole grains, lean meats, low fat dairy, fruit and vegetables) increased to eight times the equivalent price of unhealthy foods (sweets, calorific drinks and fatty foods) between 2004 and 2008 in Seattle, USA.7 Even payment methods have been implicated in unhealthy food choices: in a study in Buffalo, USA, buying of unhealthy compared to healthy foods significantly increased when the payment method was by card compared to cash (P < 0.01).8 Table 20.1 Definition of obesity according to body mass index Adapted from Dixon JB, Zimmet P, Alberti KG et al. Bariatric surgery: an IDF statement for obese Type 2 diabetes. Diabet Med 2011; 28: 628-642. The association between obesity and the metabolic syndrome of type 2 diabetes, hypertension, sleep apnoea and polycystic ovarian syndrome is well recognised.11 These, together with other harmful obesity-related comorbid disease such as non- alcoholic steatohepatosis, asthma, back and lower limb degenerative problems, cancer and depression, present a massive financial burden on health services. In addition, obese people are known to have higher unemployment and higher rates of claiming state benefit, and consume a disproportionate amount of the healthcare budget.12,13 The traditional advice ‘eat less and exercise more’ given to patients, based on understanding of the basic energy equation, does not work in the majority: a large study in the USA of 107 000 individuals using the Behavioural Risk Factor Surveillance System found that 63% of men and 78% of women were attempting to lose weight or maintain weight at any one time. However, 34% of men and 40% of women maintained the same energy intake despite reducing fats, and only 1 in 5 of those trying to control their weight were able to eat fewer calories and do 150 minutes exercise per week.14 In chronic obesity it is recognised that patients have usually missed the boat of prevention.15 Most patients are able to diet to some extent but nearly always reach a plateau; thus a typical pattern in someone who has already become obese is a lifetime of repeated dieting and weight regain. It is thus not surprising that the worldwide rate of bariatric surgery has increased dramatically. In the USA, the most obese nation, the volume of surgery was 136 000 operations in 2004, while the American Society for Bariatric and Metabolic Surgery (ASMBS) estimated that 220 000 procedures were carried out in 2008.18 This figure is similar to cholecystectomy, and at this rate of surgery the UK would be doing more than 50 000 operations per year. Worldwide the commonest bariatric procedure is the Roux-en-Y gastric bypass (RYGB). Data from the UK and Ireland National Bariatric Surgery Registry (NBSR) indicated that in 6483 operations in 2009–10, 67% of the NHS patients had a gastric bypass, 21% gastric banding and 10.5% a sleeve gastrectomy.19 In Europe the rate of gastric banding fell during the 2000s, having far exceeded gastric bypass during the 1990s.20 By contrast, the popularity for banding increased in the USA after the FDA granted approval in 2001, and currently is estimated to exceed bypass (46% vs. 44%), with sleeve gastrectomy at 7.8%.21 Type 2 diabetes is the most important comorbid disease of obesity, affecting around 280 million people, a figure that is likely to rise to 438 million by 2030.22 About 85% of type 2 diabetics are obese and the increased risk of becoming diabetic is estimated to be 93-fold for women and 42-fold for men who are severely obese.23 Medical treatment cannot stop diabetes from progressing and thus the epidemic is one of the largest threats to global health in the 21st century. Obesity is the second most common cause of preventable cancer after smoking. In data from the Cancer Prevention Study II in the USA from 1982 to 1998, the relative risk (RR) of death from cancer increased above a BMI of 30 for both men and women.24 In 900 000 people the risk of most, if not all, cancers was increased in non-smokers.25 For women with BMI > 40, there was an RR of 6.25 for uterine cancer, and the overall RR was 2.51 for other cancers, in particular kidney, cervix and pancreas. For men with BMI > 35, there was an RR of 4.52 for liver cancer, and for BMI > 30 the RR was 1.68 for all other cancers. Thus the impact of obesity appears to be important for most, if not all, organs. Up to 60% of bariatric patients have psychiatric disorders and substantial psychiatric comorbidity.26 They also have impaired self-esteem, lower quality of life, and more depression and anxiety than the general population. About one-third are victims of sexual child abuse. Anti-obesity stereotypes are common in society and also in healthcare professionals: the obese are regarded as ‘lazy, unmotivated and non-compliant’, they ‘lack self-discipline’, and they face discrimination and prejudice.26 Obesity is regarded as being ‘self-inflicted’ and is associated with ‘moral failure and guilt’, underlining the need for sensitivity and non-judgmental approaches to management. In common with the international literature, a very high proportion (80.1%) of patients undergoing bariatric surgery were female. It is not known why so few men have surgery, but men were older, heavier and had more comorbidity. For example, 43% of the men were diabetic, 50% had hypertension, 28% had dyslipidaemia and 37% had sleep apnoea (P < 0.001). The average BMI was 50.6 compared to 47.7 in females. The differences are not simply explained by the older age of males, since on age group comparison males age 40–49 had a median of three comorbidities compared to a median of two in females of the same age group (P < 0.001).19 The team should include bariatric physicians, dieticians, nurse specialists, psychologists, anaesthetists and surgeons, and support groups.29–31 Current National Institute for Clinical Excellence (NICE) guidelines, which were based on National Institutes of Health guidelines (1991), are shown in Box 20.1. It is not known what proportion of patients who fulfil the weight threshold for surgery would ever be suitable as patients must show that they are engaged and committed to the process and this is difficult to measure. Some believe that demonstration of weight loss before surgery is necessary to gauge this, but the evidence base for this is poor or lacking.28 Rare endocrine causes should be excluded. Current drug or alcohol misuse and uncontrolled psychiatric disease are regarded as contraindications, as are a ‘lack of comprehension of risks and lifestyle changes required’, which might exclude patients with learning difficulties.20,28 Patients should not be operated on when pregnant.28 Crohn’s disease is a relative contraindication and portal hypertension is an absolute contraindication.20 Multidisciplinary teams should ensure that medical comorbidities can be identified and treated, so that high-risk patients with multiple medical comorbidities can be made as fit as possible before surgery.28,30 This includes indentifying and treating obstructive sleep apnoea before surgery, and careful perioperative control of diabetes.23 It is recommended that patients stop smoking at least 8 weeks before surgery.28 Medications for epilepsy should be considered carefully as the absorption may be insufficient after gastric bypass or duodenal switch. Thus a male patient with high BMI and hypertension, both of which are associated with central obesity, is likely to be more difficult to operate on due to the extra torque on laparoscopic instruments. Usual practice, in addition to medical work-up, is to put patients on a ‘liver shrinkage diet’ for at least 2 weeks before surgery, as this has been shown to reduce liver size, and probably reduces torque in central obesity (Grade B recommendation).28,33 Weight reduction before surgery may also downgrade the risk group. There are several laparoscopic techniques described and there is as yet no standardisation. However, the weight loss results appear very much the same. Most agree that a short vertical lesser curvature-based gastric pouch of no more than 5–6 cm, that is separated from fundus, should be constructed, as this has been shown by MacLean and colleagues to produce weight loss over 15 years.2 Starting just below the oblique fat pad on the lesser curve and usually taking the second branch of the left gastric artery, the pouch is made with linear staplers directed transversely over 2–3 cm then vertically up to just to the left of the angle of His, staying to the right of the posterior gastric artery if identified. Routing of the Roux limb can be retro- or antecolic and particular attention must be paid to the correct identification of the bowel limb to prevent ‘Roux-en-O’34,35 (Fig. 20.1). Figure 20.1 Gastric bypass showing short vertical lesser curve-based gastric pouch with Roux-en-Y jejuno-jejunostomy. In open surgery exact calibration of the diameter of the anastomosis of the Roux limb to the gastric pouch was thought to be critical to the rate of emptying and thus weight loss after the operation. However, as for banded gastric bypass (Fobi/Capella), there were no good data to support this.36,37 In fact, an early study by Naslund in Sweden of 29 patients found that between-patient variation in the size of the gastric outlet did not correlate with weight loss 1 year after bypass.38 In the UK NBSR, the techniques of pouch-enterostomy are reported as 39% linear stapler with suture closure of the defect (after Lonroth), 23% circular stapler (after Wittgrove) and 38% hand sewn(after Higa).19,34,35,39 The lengths of the biliary and Roux limbs are not standardised and are impossible to measure accurately but in the US literature the biliary limb length is not usually mentioned since the assumption is that it is ‘kept short’ in order to reduce malabsorption of vitamins and minerals. The commonest description of the Roux limb is around 100–150 cm, after Brolin et al. in New Jersey and MacLean and colleagues in Montreal.2,40 Limited evidence suggests that a longer Roux limb of 150 cm gives more weight loss for BMI > 50, but this is not maintained in the long term. In standard bypass protein/calorie malabsorption is not the mechanism of weight loss. Since the common channel length is the determinant of available gut for absorption, and this is never measured in standard bypass, it is unlikely that longer lengths will ever be studied because of the worse nutritional consequences that would ensue.41 The early perigastric approach for tunnelling the band posteriorly has now been abandoned as it is associated with high rates of band erosion into the stomach. The pars flaccida technique (through the window of the lesser omentum) is now preferred, keeping above the lesser omental bursa posteriorly. A band placed around the gastro-oesophageal junction would produce weight loss by dysphagia, and correct placement is just below, producing a small ‘virtual pouch’ of gastric mucosa above the band20 (Fig. 20.2). The access port is usually sutured to the rectus sheath in the upper abdomen for ease of access by a non-coring, Huber needle for band adjustments. Placement of the band is just the start of the process of weight loss. Adjustments of the band are made by injecting saline progressively in order to reach the so-called ‘sweet spot’ of optimal restriction. A patient should probably be offered monthly clinic visits, at least for the first year. One study found that patients who attended seven or more clinics achieved 50% EWL in the first year compared to only 42% EWL in those who attended six times or fewer (P < 0.01).42 The ability to provide good follow-up should be part and parcel of the process, but is often lacking.20 Technically simpler than gastric bypass, the operation evolved from the Magenstrasse and Mill operation described by Johnston in Leeds, where the divided fundus (the ‘mill’) was left in continuity with the lesser curve-based tube (the ‘main street’).3 In the sleeve the lesser curve-based tube is constructed over a size 32 or 34Fr bougie. The dissection uses linear stapling devices and starts 3–6 cm proximal to the pylorus upwards to just lateral to the angle of His.43 Some descriptions of the sleeve now remove more of the antrum, leaving a true sleeve upwards from the pylorus (Fig. 20.3). Sleeve gastrectomy is seen as a less risky alternative to gastric bypass, and a number of trials have now been reported (see below). The ASMBS issued an update statement on sleeve gastrectomy in October 2011, summarising the current data.44 Himpens et al. from Belgium have the largest published series, with 5-year follow-up in which they found that 30 sleeve patients had 77.5% EWL at 3 years and 53.3% EWL at 6 years.45 However, the starting BMI was only 39.9 and an additional 11 patients had a further bariatric procedure during follow-up, including ‘re-sleeve’ because of weight regain. Although there is much enthusiasm for sleeve gastrectomy, the lack of long-term studies and demonstrable superiority compared to both gastric bypass and banding, together with the concern about weight regain due to expansion of the sleeve, mean this operation is unlikely to replace either in the foreseeable future. The longest follow-up so far reported is 8–9 years, when 13 patients with an initial BMI of 45.8 had 68% EWL.46 Although the biliopancreatic diversion (BPD) described by Scopinaro in Naples produces greater weight loss than gastric bypass or banding, it requires very careful nutritional follow-up due to malabsorption, which is the mechanism of action of the operation.31 BPD appears to be done rarely; however, its duodenal switch (DS) variant, combined with sleeve gastrectomy, is performed in a few centres.47 DS is increasingly seen as a rescue operation for weight regain after sleeve gastrectomy.45 All patients after DS need a high-protein diet and regular vitamin and mineral supplements with monitoring for life. The DS variant of the BPD involves a sleeve gastrectomy followed by division of the duodenum just distal to the pylorus. The ileum is divided with a linear stapler and a duodeno-ileostomy and ileo-ileostomy are made such that the common channel for food absorption measures 75–125 cm and the alimentary channel measures 100–250 cm. The long remaining biliary limb is not measured47 (Fig. 20.4). Both bypass and banding appear to control appetite and satiety by physiological mechanisms. In a blinded crossover trial of filling/de-filling of gastric bands, Dixon et al. demonstrated that satiety before and after eating was greater when the band was filled than when not.48 It is proposed that stimulation of vagal afferent fibres adjacent to the band mediates the feeling of satiety, as there is no marked change, if any, in gut hormone profiles.20 In contrast to banding, there has been an explosion of publications demonstrating rises in gut hormone levels after gastric bypass that favour reduced appetite and early satiety. Hormones such as peptide YY (PYY), glucagon-like peptide-1 (GLP-1) and oxyntomodulin have attracted particular attention and there are currently publications exploring changes in energy expenditure and bile acids as mediators of improved insulin resistance.49,50 In the foregut theory, diabetes has been proposed to be a disease of the duodenum and proximal jejunum, referring to the exclusion of this part of the anatomy to the passage of food in gastric bypass. In the original research, diabetic rats were made non-diabetic after duodeno-jejunal bypass, in which only the duodenum and proximal jejunum were excluded from the passage of food, leaving the stomach intact. This is the basis for the development of the duodeno-jejunal bypass in humans and the endoscopic duodenal sleeve technique (Endobarrier).51 If current trials show success, the endoscopic sleeve could become a useful adjunct in making patients fitter for surgery. In the distal gut theory, rapid delivery of food from the gastric pouch via the Roux limb to the terminal ileum is proposed as the mechanism by which gut hormones rise and produce early reduction in appetite and early satiety.49 In this study, patients with good weight loss produced high levels of PYY and GLP-1 compared to patients who did not lose as much weight (P < 0.05). In addition, in a randomised controlled trial (RCT) those receiving somatostatin after gastric bypass had attenuated gut hormone responses and experienced return of appetite compared to those receiving placebo (P < 0.05).49 The distal gut theory contradicts the thinking behind the banded bypass. A measurement of weight loss is the most consistent variable used in reporting results of bariatric surgery, being regarded by surgeons as the paramount clinical outcome. There is, however, almost complete lack of consistency about how this is measured or presented, as illustrated in Table 20.2, in which the known RCTs of weight loss outcomes from currently performed procedures are summarised.52 This lack of consistency leads to difficulties in combining data from studies in systematic reviews and meta-analyses, and it makes comparisons between studies and centres difficult. Most of the available evidence consists of case series (level III evidence). Table 20.2 Summary of RCTs of currently performed procedures52 *Added in addition to Padwal systematic review. Two RCTs have compared gastric bypass and adjustable gastric banding. Angrisani et al. in Naples randomised 24 patients to gastric bypass and 27 patients to banding.62 There was no mortality and one band patient was lost to follow-up. Four band patients required revision to other bariatric operations during follow-up, two for gastric pouch dilatation and two for unsatisfactory weight loss. Three bypass patients required early re-operations because of life-threatening complications (12.5%). Fifteen bypass patients (62.5%) achieved BMI < 30 compared to three (11.5%) band patients (P < 0.001). The authors concluded that gastric bypass produces better weight loss and fewer failures, acknowledging that the high complication rate was accounted for by their learning curve in the bypass operation. Nguyen et al. in California randomised 111 patients to gastric bypass and 86 to banding.63 There was no mortality in either group. At 4 years patients were grouped into quintiles of percentage excess weight loss (% EWL). The highest quintiles representing 60% EWL or more had 64.1% of bypass patients and 15.3% of band patients. The lowest quintiles representing < 40% EWL had 5.1% of bypass patients and 50.0% of band patients (P < 0.05). Although the authors concluded that weight loss at 4 years was superior after bypass, the follow-up rates achieved were only 83.1% for bypass and 93.3% for band, and no explanation was given for the different numbers randomised to each arm. In the largest RCT, Himpens et al. in Belgium randomised 80 patients to either operation.54 Weight loss was better for sleeve at each time point measured: 57.7% EWL vs. 41.4% EWL at 1 year and 66% EWL vs. 48% at 3 years (both P < 0.01). Although there were several operations for complications in each group, an additional two patients in each was converted to another bariatric operation due to lack of weight loss.
Bariatric surgery
Introduction
Obesity as a public health problem
BMI (kg/m2)
WHO classification
Common clinical description
18.5–24.9
Normal range
Desirable
25–29.9
Pre-obese
Overweight
30–34.9
Obese class I
Obese
35–39.9
Obese class II
Clinically severe and complex obesity
40–49.9
Obese class III
50 and over
Super-obesity
Diabetes risk with obesity
Cancer risk with obesity
Psychosocial morbidity and prejudice
Baseline obesity-related disease
Data from the NBSR
Multidisciplinary work-up
Optimisation of patients
Obesity Surgery–Mortality Risk score (OS-MRS)
Current bariatric operations and surgical techniques
Gastric bypass
Gastric banding
Sleeve gastrectomy
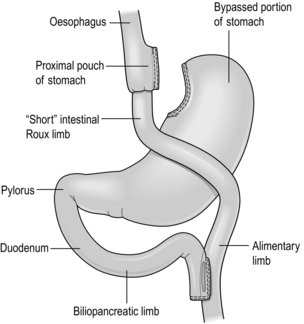
Biliopancreatic diversion/duodenal switch
Mechanisms of bypass and banding
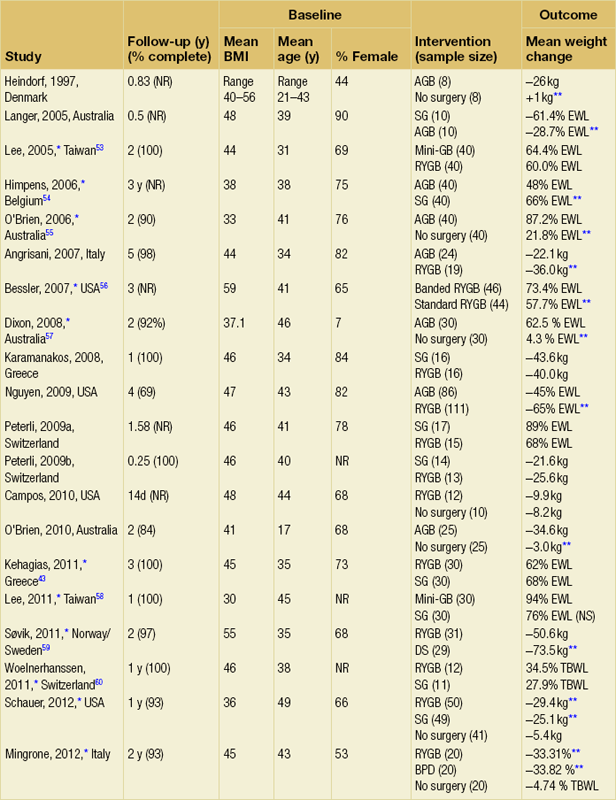
Weight loss outcomes
Band versus bypass RCTs
Band versus sleeve gastrectomy RCT
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Abdominal Key
Fastest Abdominal Insight Engine