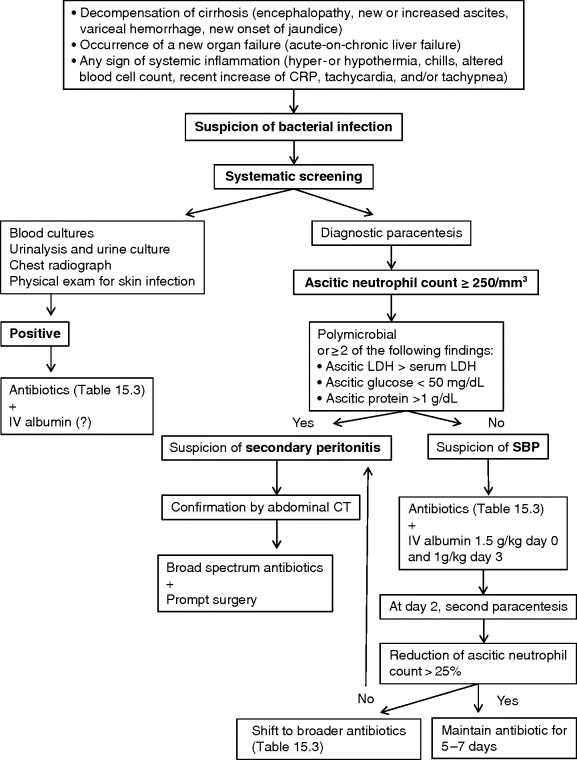
Chapter 15 Thierry Gustot1,2,3 and Richard Moreau4,5,6 1Department of Gastroenterology and Hepato-Pancreatology Erasme Hospital Brussels Belgium 2Laboratory of Experimental Gastroenterology Université Libre de Bruxelles Brussels Belgium 3INSERM U773, Centre de Recherche Biomédicale Bichat-Beaujon CRB3 Paris France 4INSERM, U1149, Centre de Recherche sur l’Inflammation (CRI), Paris, France 5UMR S 1149, Université Paris Diderot-Paris 7, Faculté de Médecine Bichat, Paris, France 6Département Hospitalo-Universitaire (DHU) UNITY, Service d’Hépatologie, Hépital Beaujon, Assistance Publique-Hôpitaux de Paris, Clichy, France In cirrhosis, bacterial infection is defined as a pathologic process caused by invasion of normally sterile tissue, fluid, or cavity by pathogenic or potentially pathogenic bacteria. Bacterial infection is present at admission or develops during hospitalization in about 30% of patients with cirrhosis [1]. Infections may be classified as follow: Table 15.1 Types of bacterial infection. Approximatively one-third of bacterial infections are community-acquired, one-third healthcare-associated, and one-third nosocomial. Spontaneous bacterial peritonitis (SPB) and urinary tract infections (UTI) are the most frequent infections observed, followed by pneumonia, skin and soft tissue infections (SSTI), spontaneous bacteremia and catheter-related infections [3]. Clinical risk factors associated with occurrence of bacterial infections in cirrhosis are high Child–Pugh score, variceal bleeding, low ascitic protein levels, and prior episode of SBP [4–7]. Infection induces a systemic inflammatory host response with three stages of severity: sepsis, severe sepsis (when an acute organ failure occurs), and septic shock (when hypotension does not respond to adequate fluid resuscitation). Patients with cirrhosis have increased risk to develop bacterial infection, sepsis, sepsis-induced organ failure, and death [8]. A recent prospective European study defined the acute-on-chronic liver failure syndrome characterized by organ failures (defined by an adapted Sequential Organ Failure Assessment (SOFA) score, the Chronic Liver Failure (CLIF)-SOFA score; Table 15.2), requirement of intensive care management and high 28-day mortality (between 23% and 74%) [9]. The mortality of infected patients with cirrhosis reaches 38% [10]. Cirrhotic patients are two times more likely to die from sepsis than individuals without cirrhosis [11]. In-hospital mortality of cirrhotic patients with severe sepsis without shock or with septic shock is 41% and 71%, respectively [12]. Table 15.2 Definition of organ failures in patients with cirrhosis (in gray). Organ/systems whose functions are assessed by the Chronic Liver Failure (CLIF) Sequential Assessment of Organ Failure (SOFA) score. The early diagnosis of bacterial infections and the prompt initiation of adequate antibiotherapy are critical in the management of cirrhotic patients. The diagnosis of bacterial infection in cirrhosis is very difficult for several reasons. First, in the early phase of infection, cirrhotic patients may be totally asymptomatic. Second, the classic parameters assessing the inflammatory host response to infection (systemic inflammatory response syndrome (SIRS)) are not specific for the diagnosis of infection in cirrhosis. SIRS is defined as the presence of at least two of four clinical criteria: body temperature ≥38 °C or ≤36 °C, heart rate ≥90 beats/min, respiratory rate ≥20 breaths/min or hyperventilation with a PaCO2 ≤32 mmHg, white blood cell count ≥12 000/mm3, ≤4000/mm3, or with >10% immature neutrophils [13]. Decompensated cirrhosis may be associated with some degree of encephalopathy-related tachypnea, tachycardia, or hypersplenism-related leukopenia. SIRS has a low sensitivity for the diagnosis of infection (57–70% of infected patients with decompensated cirrhosis) and is not specific (10–30% of patients without infection) [14,15]. On the other hand, the presence of SIRS in decompensated cirrhotic patients is a marker of poor prognosis. Thus, SIRS is associated with higher in-hospital mortality (31% vs. 4%). Moreover, common early markers of infection used in the general population, such as C-reactive protein (CRP) and procalcitonin, are not sufficient to distinguish infected cirrhotic patients. Indeed, CRP >2 mg/dL has only a sensitivity of 78% and specificity of 68% and procalcitonin >3 ng/mL has a sensitivity and specificity of 73%. This poor diagnostic accuracy could be explained by the decreased production of acute-phase proteins by the liver, especially CRP. Particularly, low CRP values should be interpreted with caution in patients with severe liver insufficiency.Infection should be suspected in any decompensated cirrhotic patient, when a hospitalized patient deteriorates (worsening of liver function, hepatic encephalopathy, shock, renal failure, or gastrointestinal bleeding), or when there are signs of systemic inflammation (hyper- or hypothermia, chills, altered blood cell count, tachycardia, and/or tachypnea). Therefore, a complete check-up, including urinary sediment and culture, diagnostic paracentesis and ascitic fluid culture, blood culture, and chest X-ray, must be made in order not to delay diagnosis and the administration of empiric antibiotics (for the therapeutic strategy see Figure 15.1). The choice of empiric antibiotics based on the site is proposed in Table 15.3. When the bacteria has been identified, we must potentially shift to a more specific antibiotic to avoid the occurrence of antibiotic resistance. Figure 15.1 Therapeutic strategy for suspected bacterial infection. CRP, C-reactive protein; CT, computed tomography; LDH, lactic dehydrogenase; SBP, spontaneous bacterial peritonitis. Table 15.3 Empiric antibiotic therapy for common bacterial infections in cirrhosis. SBP is defined as an ascitic fluid infection without an evident abdominal source. It is the most frequent infection in cirrhotic patients (20–25% of all infections) [3]. In outpatients without symptoms the prevalence is low (<3%) [16,17] but it increases to 8–36% in hospitalized patients. The mortality for the first episode ranges from 10% to 25% [18]. Importantly, the 1-year mortality rate after the first SBP episode is reported to be at least 30%, suggesting that the deterioration of liver function accelerates [19]. Patients with SBP may have one of the following: local symptoms and/or signs of peritonitis (abdominal pain, abdominal tenderness, Blumberg sign, vomiting, diarrhea, ileus). SBP may be asymptomatic, particularly in outpatients. The diagnosis of SBP is based on ascitic neutrophil count ≥250/mm3 [20]. Ascitic fluid neutrophil count is obtained by centrifugation of the ascitic fluid and then stained with Giemsa and differential cell counts are made with an optical microscope. For faster and cheaper results, reagent strips have been assessed, but a high rate of false negative results (around 50%) prevents their use [21]. Ascitic fluid lactoferrin measurement seems to be an alternative to ascitic fluid neutrophil count in the diagnosis of SBP. Indeed, a cutoff value of 242 ng/mL had a sensitivity and specificity in the diagnosis of SBP of 95% and 97%, respectively, in one study [22]. These results must be replicated before the method can be recommended for use in clinical practice. Pleural ascites could be also a site of infection. This infection is then called spontaneous bacterial empyema (SBE) and the diagnostic criteria are the same as in SBP. Ascitic fluid cultures (10 mL into aerobic and anaerobic blood cultures), direct ascitic microscopic examination (rapid assessment for polymicrobial infection in the case of secondary peritonitis), and blood cultures (50% of SBP are associated with bacteremia) should also be obtained when SBP is suspected. The ascitic fluid culture is positive in 40% of cases. The most common pathogens are Gram-negative bacteria (GNB) (mainly Escherichia coli, Klebsiella pneumoniae, Enterobacter spp.) and Gram-positive cocci (GPC) (mainly Streptococcus spp. and Enterococci). In Spain, 20% of GNB are resistant to quinolones and 70% of these are also resistant to trimethoprim-sulfamethoxazole [1]. Long-term norfloxacin administration (see prevention section) increases the rate of quinolone resistance to 60% and the proportion of GPC. The rate of cephalosporin-resistant GNB is low in community-acquired SBP regardless of long-term norfloxacin prophylaxis. On the other hand, in Spain, extended-spectrum β-lactamase (ESBL)-producing Enterobacteriacae are isolated in 30% of nosocomial SBP and methicillin-resistant Staphylococcus aureus in 10% of healthcare-associated SBP [3]. Some patients have bacterascites in which cultures are positive but there is an ascitic neutrophil count of <250/mm3. When this bacterascites is monomicrobial, it usually represents the colonization phase of ascitic fluid infection. It may progress to SBP or, in the majority of cases (62–86%), resolve spontaneously [23,24]. Bacterascites with microorganisms similar to those of the skin flora are probably due to contaminants. If symptoms are present, a second paracentesis is recommended. For community-acquired SBP, third-generation cephalosporins (cefotaxime, ceftriaxone) are the gold standard for empirical antibiotic treatment (Table 15.2
Spontaneous Bacterial Peritonitis and Other Infections
General Considerations
Type
Definition
Community-acquired infection
Diagnosis at the admission or during the first 48 hours after hospitalization
Nosocomial infection
Diagnosis after 48 hours of hospitalization
Healthcare associated infection
Diagnosis at the admission or during the first 48 hours after hospitalization with an history of previous contact with a healthcare environment (hospitalization or short-term admission for at least 2 days in the previous 90 (−180) days, residence in a nursing home or a long-term care facility, or chronic hemodialysis)
Organ/system
0
1
2
3
4
Lungs
(PaO2/FiO2)
or SpO2/FiO2
>400
>512
≤400
>357–≤512
≤300
>214–≤357
≤200
>89–≤214
≤100
≤89
Coagulation
INR
<1.1
1.1–1.25
1.26–1.5
1.51–2.5
>2.5
or platelets ≤20 × 103/μL
Liver
Bilirubin, mg/dL
<1.2
1.2–1.9
2–5.9
6–11.9
>12
Circulation
MAP (mmHg)
≥70
<70
Dopamine ≤5 or dobutamine or terlipressin
Dopamine >5, epinephrine ≤0.1, or norepinephrine ≤0.1
Dopamine >15, epinephrine >0.1, or norepinephrine >0.1
Cerebral
HE grade
No HE
1
2
3
4
Kidney
Creatinine (mg/dL)
<1.2
1.2–1.9
2–3.4
3.5–4.9
≥5
Adrenergic agents were administred for at least 1 hour (doses are expressed as μg/kg per minute).
FiO2 denotes fraction of inspired oxygen.
HE, hepatic encephalopathy; MAP, mean arterial pressure.
The Diagnostic Approach
Sites
Types
Antibiotics
SBP, SBE, and spontaneous bacteremia
Community-acquired
Cefotaxime (2 g/6 h or 2 g/12 h IV)
Ceftriaxone (1 g/24 h IV)
Amoxicillin/clavulanic acid (1–0.2 g/8 h then 0.5–0.125 g/8 h PO)
Nosocomial
Piperacillin/tazobactam (4 g/6 h IV)
Meropenem (1 g/8 h IV)a
UTI
Community-acquired
Ciprofloxacin (500 mg/12 h PO) or cotrimoxazole (160–800 mg/12 h PO) if uncomplicated UTI
Cefotaxime (2 g/6 h or 2 g/12 h IV) or Amoxicillin/clavulanic acid (1–0.2 g/8 h IV) if sepsis
Nosocomial
Nitrofurantoin (50 mg/6 h PO) if uncomplicated UTI
Piperacillin/tazobactam (4 g/6 h IV) or Meropenem (1 g/8 h IV)a if sepsis
Pneumoniab
Community-acquired
Amoxicillin/clavulanic acid (1–0.2 g/8 h IV) or ceftriaxone (1 g/24 h IV) and macrolide or levofloxacin (500 mg/24 h PO)
Or moxifloxacin (400 mg/24 h PO)
Nosocomial
Piperacillin/tazobactam (4 g/6 h IV) or Meropenem (1 g/8 h IV)a + vancomycine (15 mg/kg/12 h IV) or teicoplanin (first dose 18 mg/kg followed by 6 mg/kg/24 h IV) in patients with risk factors for MRSA
SSTI
Community-acquired/nosocomial
Amoxicillin/clavulanic acid (1–0.2 g/8 h IV) or Ceftazidim (2 g/8 h IV) in patients with risk factors of Pseudomonas spp. or meropenem (1 g/8 h IV)a
+ Oxacillin (2 g/6 h IV); or vancomycine (15 mg/kg/12 h IV) or teicoplanin (first dose 18 mg/kg followed by 6 mg/kg/24 h IV) in patients with risk factors for MRSA
aIn nosocomial infections in geographic areas with high prevalence of ESBL-producing bacteria.
bLiver disease is considered as severe comorbidity for community-acquired pneumonia in guidelines.
ESBL, extended-spectrum β-lactamase; MRSA, methycillin-resistant Staphylococcus aureus; SBE, spontaneous bacterial empyema; SBP, spontaneous bacterial peritonitis; SSTI, skin and soft tissue infections; UTI, urinary tract infection.
Spontaneous Bacterial Peritonitis
Diagnosis
Microbiology
Antibiotics
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







