Chapter 12 Jian-Gao Fan and Hai-Xia Cao Department of Gastroenterology, Shanghai Key Laboratory of Pediatric, Gastroenterology and Nutrition, Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine Shanghai China Malnutrition is a condition that results from an unbalanced diet in which certain nutrients are lacking, in excess (too high an intake), or in the wrong proportions. There are several types of malnutrition including undernutrition and obesity, depending on which nutrients are under or overabundant in the diet (Table 12.1). Because the major form of malnutrition in patients with cirrhosis is undernutrition or protein–calorie malnutrition, malnutrition in the current chapter only refers to protein–calorie malnutrition. Malnutrition is a frequent and integral component of acute and chronic diseases. Patients with advanced liver disease commonly have malnutrition but its assessment is confounded by many of the usual indicators of nutritional status. Although a clear consensus on the criteria of malnutrition in cirrhosis is still lacking at present, malnutrition is extremely common in cirrhotic patients worldwide. Table 12.1 Classification of malnutrition. The working definition of malnutrition in cirrhosis in most studies is decreased lean body mass as well as diminished skeletal muscle weight and reduced fat mass. On one hand, malnutrition is the single reversible prognostic marker in cirrhosis that accelerates deteriorating liver function and adversely affects clinical outcome both before and after liver transplantation, including increased morbidity and prolonged hospital stay [1,2]. Mortality increases in direct proportion to the extent of malnutrition, approaching 80% in patients with severe malnutrition (i.e., less than 50% of normal body weight) [3]. On the other hand, adequate nutrition is a fundamental component of survival and quality of life in cirrhotic patients, and correction of malnutrition might improve the clinical outcome of these patients. Therefore, nutritional assessment should be routinely performed in cirrhotic patients, and aggressive nutritional management might be useful in patients with malnutrition. However, the mechanism leading to malnutrition is not fully understood yet, and the logistic challenges of identifying patients in need of nutritional therapy and determining the type, duration, and quantity of nutritional supplements remain problematic. Malnutrition, characterized by protein and calorie deficiency, is defined as a state of nutrition in which a deficiency of energy, protein, and other nutrients causes measurable adverse effects on tissue and body form and function and on clinical outcome (Table 12.2). There are three forms of malnutrition: kwashiorkor (protein malnutrition predominant), marasmus (deficiency in calorie intake), and marasmic kwashiorkor (both marked protein deficiency and calorie insufficiency signs are present). Marasmic kwashiorkor is sometimes referred to as the most severe form of malnutrition and is more commonly seen in the context of a wide variety of diseases that lead to depletion of body fat, muscle wasting, and multiple signs of micronutrient deficiencies. Table 12.2 The usual indicators of protein–calorie malnutrition. As the liver is the most important metabolic organ that regulates a complex array of physiologic and biochemical processes including protein and energy metabolism, malnutrition may commonly be secondary to chronic liver disease, especially advanced cirrhosis in which protein energy wasting may occur. Malnutrition in patients with cirrhosis is multifactorial (Table 12.3), including reduced food intake, maldigestion, and malabsorption. Many of these factors can be treated successfully if the nutritional status is recognized. Table 12.3 Factors contributing to malnutrition in cirrhosis. The majority of cirrhotic patients have inadequate dietary intake which is attributed to several factors. Anorexia is a frequent symptom in patients with cirrhosis, with an incidence as high as 90% [4]. Many patients with advanced liver disease have an altered sense of taste, which might be related to vitamin A and/or zinc deficiency [5]. Patients with cirrhosis often experience early satiety that is related to mechanical compression from massive ascites [6]. Patients with chronic liver diseases experience abdominal pain, nausea, and bloating [7], and are found to have altered gut motility [8], all of which lead to the development of functional dyspepsia. The dietary restrictions that are commonly recommended to these patients, such as restriction of sodium, protein, and water, can discourage adequate oral intake. In addition, weakness, fatigue, and low-grade encephalopathy can contribute to decreased oral intake. Other iatrogenic causes for protein and caloric loss include multiple hospitalizations which may lead to loss of regular meals for reasons of pending examinations and procedures. Cholestatic liver disease is one of the reasons for impaired absorption, especially of fat-soluble vitamins such as A, D, E, and K, due to reduced intraluminal bile salt concentrations [9,10]. Chronic alcohol drinking impairs gastrointestinal mucosal absorption of nutrients, especially folate and vitamin B12. Furthermore, conditions such as small intestine bacterial overgrowth, coexistent small intestinal disease (i.e., inflammatory bowel disease and celiac sprue), pancreatic insufficiency, mucosal congestion, and villus atrophy contribute to the impaired absorption and utilization of nutrients [8,11,12]. The presence of portal hypertension has also been implicated as a cause of malabsorption and gastrointestinal protein loss [13,14]. An additional factor is the administration of medications that lead to malabsorption, such as neomycin, which has been used in the treatment of hepatic encephalopathy [15]. Other important factors in the loss of body protein are the inadequate synthesis of various proteins by the affected liver. It has been observed that among cirrhotic patients, an early switch to gluconeogenesis from amino acids originating from body proteins after an overnight fast is often the rule. Deficiency in vitamins and trace minerals is often observed in advanced cirrhosis. The most common iatrogenic interventions are the use of diuretics in order to cope with ascites and fluid retention. Last but not least, the occult or overt blood loss from esophageal and gastric varices and the intestinal lumen due to ulcerations or portal enteropathy are some of the main reasons for protein loss [16]. Another factor that might contribute to malnutrition, around which there has been considerable debate, is increased energy expenditure. The well-recognized hyperdynamic circulation in cirrhosis is associated with a systematic vasodilatation and an expanded intravascular blood volume leading to an increased cardiac stroke volume. Therefore, greater use of macro- and micro-nutrients is one of the most common causes of high energy demand and expenditure. In fact, it has been suggested that up to 30% of patients with cirrhosis are actually hypermetabolic [17–19]. Malnutrition is common in patients with cirrhosis and increase the severity of disease [20,21]. Therefore, every hospitalized patient should have an assessment of their nutritional status. Severely malnourished patients have poorer outcomes, regardless of the disease entity or reason for hospitalization. The usual evaluation of malnutrition includes the following. In 2006, the European Society for Parenteral and Enteral Nutrition (ESPEN) suggested a simple and practical method to assess nutritional status. The methods that are universally used to evaluate nutritional status and to detect the presence of malnutrition are SGA, and some anthropometric parameters and laboratory tests [22]. The algorithm for assessing the nutitional state is seen in Figure 12.1. Figure 12.1 Algorithm for assessing nutritional state in cirrhotic patients. BMI, body mass index; MAMC, mid-arm muscle circumference; NRS, nutrition risk screening; SGA, subjective global assessment; TST, tricep skinfold thickness. SGA is recommended by ESPEN as a practical bedside method that combines multiple elements of nutritional assessment to classify the severity of malnutrition [22]. These components are weight loss during the previous 6 months, changes in dietary intake, gastrointestinal symptoms, functional capacity, metabolic demands, signs of muscle wasting, and the presence of presacral or pedal edema. The SGA is scored from 0 to 3 as well nourished (0), mild malnutrition (1), moderate malnutrition (2), and severe malnutrition (3). Of the various components, muscle wasting is weighted the most [23,24], and the change of body weight is the least reliable because it can be confounded by the fluid retention frequently seen in these patients. As it calculates recent body weight loss or gain, the SGA is considered reliable, and it is not affected by fluid retention or the formation of ascites. The SGA has been shown to have an interobserver reproducibility rate of 80% [25,26]. Apart from the data that are collected by the SGA questionnaire, the ESPEN guidelines recommend the use of simple anthropometric parameters in evaluating malnutrition which are also not affected by the presence of ascites and peripheral edema. These parameters consist of body mass index corrected for fluid retention (BMIc), mid-arm muscle circumference (MAMC) or mid-arm circumference (MAC), and triceps skinfold thickness (TST). However, it is suggested that they should be performed by experienced clinicians in order to avoid intra- and interobserver variabilities. The BMIc is calculated as estimated dry body weight/(height)2 (in kg/m2). The BMIc cutoff values are used to indicate malnutrition, for example, 22, 23, and 25 kg/m2 in patients without, with mild, and with tense ascites, respectively. Diagnosis of malnutrition is established on values of MAMC and/or TST below the fifth percentile in patients aged 18–74 years, or the tenth percentile in patients aged over 74 years [27]. The assessment of muscle function measuring hand-grip strength and respiratory-muscle strength has also been used in nutritional evaluation; these are more useful when taken serially. The visceral proteins such as albumin, pre-albumin, retinol-binding protein, and transferrin are produced in the liver and correlate better with severity of the underlying liver disease than with malnutrition status [28]. As we know, the laboratory markers of malnutrition are capricious. Hypoalbuminemia may result from protein–calorie malnutrition but is equally likely to result from fluid shifts in recumbent and overhydrated patients; from losses in the urine, gastrointestinal tract, or the third space; and from cytokine effects or inflammation that reduces hepatic albumin production. Similarly, lymphopenia has causes other than malnutrition, particularly the corticotropin and corticosteroid response to acute biologic stress. Serum creatinine confounded by renal function may not be an accurate reflection of muscle mass in patients with renal insufficiency. Body cell mass is a useful estimation of nutritional status. The assessment techniques include bioelectrical impedance analysis (BIA) for determination of fat-free mass, dual-energy X-ray absorptiometry (DEXA) for bone mineral density and fat-free mass, in vivo neutron activation analysis for assessment of total body nitrogen, and deuterium oxide dilution for determination of total body water [29,30]. The BIA is a more readily available tool for estimating body cell mass; it is reliable in many patient populations. Furthermore, the BIA-derived phase angle is also a useful evaluating tool in chronic liver diseases [31]. However, the accuracy of BIA in patients with cirrhosis can be affected by fluid retention [32] and it does not accurately reflect changes in body composition associated with chronic liver disease. Although abnormalities of nutritional parameters are highly prevalent among patients with end-stage liver disease, most parameters of nutritional status do not correlate with body cell mass. Quantification of body composition changes in cirrhosis requires the use of direct methods such as in vivo neutron activation analysis, DEXA, or deuterium oxide dilution. However, these techniques are cumbersome, expensive, and not routinely used in clinical practice. In addition, MAMC and hand-grip strength might be the most sensitive markers of body cell mass depletion in patients with end-stage liver disease, so we prefer these simple and easy measurements. On one hand, malnutrition is often underdiagnosed in cirrhosis, as advanced liver disease – especially with ascites and edema – can affect the results of many of the traditional techniques currently used to evaluate nutritional status. On the other hand, clinically obvious malnutrition is uncommon in the pre-cirrhotic and early stages of cirrhosis, and the prevalence of obesity and the metabolic syndrome has increased sharply in these patients in the past decades. Moreover, obesity is an important risk factor for the progression of nonalcoholic steatohepatitis (NASH) and risk for the development of cirrhosis and hepatocellular carcinoma in patients with alcoholic and viral liver diseases. However, malnutrition is frequently seen in decompensated cirrhosis or end-stage liver disease. The reported prevalence in cirrhotic patients varies widely (from 20% to 100%), mainly depending on the methods used for nutritional assessment in studies and the severity of cirrhosis (Table 12.4) [33–38]. There is a direct correlation between the severity of liver disease defined by the Child–Turcotte–Pugh score and the prevalence of malnutrition. Malnutrition commonly develops in all forms of cirrhosis irrespective of the etiology of disease, and there is no difference in the prevalence of malnutrition between alcoholic and nonalcoholic cirrhosis [39–42]. Table 12.4 Prevalence of malnutrition in cirrhotic patients in different countries.
Malnutrition and Nutritional Support
Introduction
Form
Interpretation
Undernutrition
Underweight, protein–calorie malnutrition
Kwashiorkor
Selective protein malnutrition with edema and fatty liver
Marasmus
Deficiency in calorie intake with loss of body fat and protein
Marasmic kwashiorkor
Marked protein deficiency and marked calorie insufficiency signs present
Obesity
Overweight, central obesity
Etiologies of Malnutrition in Cirrhosis
If the patients meet one or more of the following criteria, they should be considered at risk for malnutrition:
Unintentional loss >10% of usual body weight in the preceding 3 months
Body weight <90% of ideal for height
BMI <18.5 kg/m2
BMI, body mass index (the weight in kilograms divided by the height in square meters).
Outpatients with cirrhosis
Inpatients with cirrhosis
Decreased diet intake
Fasting status and accelerated starvation
Decreased assimilation of diet
Use of either neomycin or lactulose for hepatic encephalopathy
Routine or prophylactic protein restriction
Stress or critical acute illness (hypermetabolism)
Abnormal metabolism of protein and energy
Effects of chronic alcoholism on protein and energy metabolism
Decreased Nutrient Intake
Malabsorption
Inadequate Synthesis of Protein
Hypermetabolic State
Nutritional Assessment in Cirrhosis
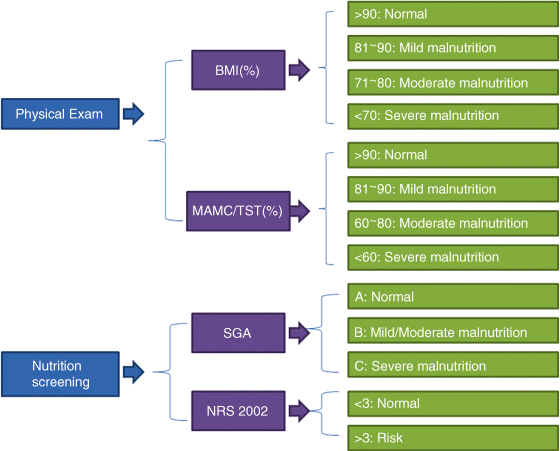
Subjective Global Assessment
Anthropometric Parameters
Laboratory Tests
Specialized Procedures for Nutritional Assessment
Prevalence of Malnutrition in Cirrhosis
Author
Year
Country
No.
Malnutrition (%)
Malnutrition in Child A
Malnutrition in Child C
P-value
Huisman
2011
Netherlands
84
56 (67%)
57%
100%
0.047
Houissa
2010
Tunis
44
35 (79.5%)
66.6%
100%
0.003
Castellanos
2008
Spain
121
55 (45%)
21%
90%
<0.001
Carvalho
2006
Brazil
300
226 (75.3%)
21%
58%
<0.05
Guglielmi
2005
Italy
334
83 (25%)
16%
44%
<0.05
Maio
2004
Portugal
117
74 (63%)
44%
68%
>0.05
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







