Center/author
Time
Recipients
Result
Hanover/Pichlmayr
February 1988
Left liver: 2 years/biliary atresia
Right liver: 63 years/primary sclerosing cholangitis
Re-transplant 4 months later
Postoperative survival 12 years
Paris/Bismuth
May 1988
Left liver: 45 years/acute liver failure
Right liver: 55 years/acute liver failure
Died 20 days after operation
Died 40 days after operation
Chicago/Broelsch
July 1988
Left liver: March/acute liver failure
Right liver: July/antitrypsin deficiency
Died 2 days after operation
Postoperative survival 12 years
Brussels/Otte
November 1988
Left liver: 5 years/tyrosinemia
Right liver: 55 years/end-stage liver disease
Postoperative survival 12 years
Died 3 days after operation
With respect to in vitro cleavage technology, Broelsch and Busuttil developed donor in situ separation technology. The keys to this technology were isolating the liver before the blocking and cold perfusing the aortic artery of brain-dead donors [10]. The in situ separation technique shortens the cold ischemia time, simplifies the identification of biliary and vascular structures, and reduces bleeding after reperfusion. In addition, this technique laid the technical foundation for living-donor liver transplantation. Of course, there were more difficult requirements for surgeons performing in situ separation because this procedure is longer than the isolated liver surgery; this factor also puts higher demands on the donor hospital. In addition, brain death must be recognized by the local legislative body. With respect to the recognition of brain death, liver-splitting surgery can only be performed legally on donors with heartbeat. In China, due to legislation failures related to brain death, only the traditional in vitro cleavages can be performed.
34.2 Anatomical Basis of Split-Liver Transplantation
The Couinaud liver segmentation system is the basis for the split-liver transplantation as a surgical procedure. This system divides the liver into eight segments that are supplied by independent parts of the Glisson system. By splitting the liver along different anatomical landmarks, two different sizes of donated liver tissue can be obtained, each with independent blood supplies and drainage tubes. Splitting the liver along the falciform ligament results in a smaller left lateral lobe (II + III, left lateral lobe, LLL) and a larger right trefoil lobe (I, IV–VIII, extended right lobe, ERL) for pediatric and adult grafts, respectively. In contrast, splitting the liver along the hepatic vein results in two similar-size donor tissues. The left lobe tissue (I–IV, left lobe, LL) can be used for a smaller adult body transplant, and the larger right lobe (V–VIII, right lobe, RL) can be used as a larger-size human liver (Table 34.2).
Table 34.2
The graft and the approximate size obtained by splitting according to different anatomical landmarks
Liver splitting | Graft | Volume (ml) | Recipient |
|---|---|---|---|
Along the falciform ligament | Left lateral lobe (II + III) | 200 | Child |
Right trefoil lobe (I, IV–VIII) | 1,000 | Adult | |
Along the hepatic vein | Left liver with caudate (I–IV) | 400 | Adult |
Right liver (V–VIII) | 800 | Adult | |
Left liver (II–IV) | 300 | Child | |
Right liver with caudate (I, V–VIII) | 800 | Adult |
In contrast to living-donor liver transplantation, splitting the liver can cause ischemia or preservation injury. Thus, recipients require a GRWR that is most likely higher than 0.8 % for living-donor liver transplantation.
34.3 The Choice of the Donor
Donor evaluation and carefully selected recipients are keys to successful split-liver transplants. Split-liver donors should generally meet the following conditions: age from 10 to 40 years, normal or below normal body weight, ABO blood type matched, normal liver function, hospitalized for a shorter time, normal basic hemodynamics, no significant hypotension, and matched size for a recipient [11–13]. Although the preoperative evaluation of these factors cannot absolutely guarantee the safety of the recipients, a conservative safety assessment can minimize the possibility of primary graft dysfunction and delayed function. Evaluating the organ after the operation is also very important both to assess the function of the liver parenchyma and to perform the anatomic analysis of the vasculature and bile duct.
34.4 The Choice of the Recipients
An accurate estimation of the liver volume that is required for the recipients after the transplant is a key factor for recipient selection. The primary disease of the recipients, the degree of illness severity, and portal hypertension are also factors that must be considered. Under normal circumstances, the weight of the liver is approximately 5 % of the weight of an infant, and this ratio gradually decreases and stabilized in adults to no more than 2.5 %. The liver weight of males is generally larger than for females. For normal livers, the liver can still regenerate following a right trisegmentectomy (only 20 % residual liver tissue). Chronic hepatitis, cirrhosis, and fatty liver will delay or hinder the process of liver regeneration.
There are two common means of assessing matched volume of the donor and recipient. The first is referred to as standard volume (standard liver volume), which is the ratio of liver donor volume to liver volume required by the recipient. The second is GRWR, which is the weight ratio of the graft and recipient. Briefly, the volume of the graft should be 40 % of the expected liver weight or at least 1 % of the patients’ weight. Donations below this standard often result in SFSS, causing delayed recovery of liver synthetic function or cholestasis syndrome. In addition, the preoperative state of the recipients has a very large impact on the postoperative outcome. Poor prognostic risk factors of split-liver transplant recipients include the following: a preoperative recipient model for end-stage liver disease (MELD) score higher than 30 points, re-transplant patient, a graft ischemia duration that is too long, and a transplant that occurs at an inexperienced center [7].
34.5 Surgical Methods
34.5.1 Donor Acquisition
In vitro liver splitting is the same as the traditional method for obtaining a donor liver. Before splitting the donor liver, conventional abdominal surgery is required. This procedure requires the following: sufficiently long enough abdominal incision, exposure of the retroperitoneal colon and duodenum structures, inferior mesenteric vein catheter placement to alternate mild cold perfusion via the portal vein, and celiac control over the abdominal aorta and the renal artery. These steps are necessary, so once the donor situation is unstable, abdominal aortic occlusion and catheter perfusion through the abdominal aorta can quickly result, allowing for rapid graft acquisition and a shortened warm ischemia time. The incisions are similar to the steps for a living donor, with the difference that the donor inferior vena cava must be cut. This step may require trimming of the hepatic vein grafts for a vascular flap. This flap is beneficial for the graft with respect to outflow tract reconstruction.
34.5.2 Splitting the Left Lateral Lobe (SII + III) and Extended Right Lobe (SI, IV–VIII)
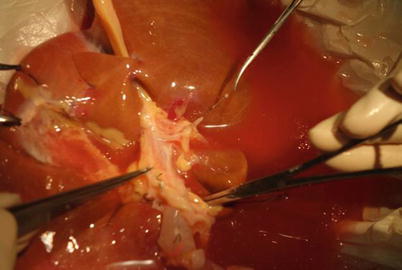
First, identify where the inferior vena cava and hepatic vein combine; then, dissect the left hepatic vein and remove the vascular leash. This step will help confirm the final site of the splitting of the liver parenchyma. The left hepatic vein and hepatic vein often have a common trunk. These veins must be isolated in the liver parenchyma after the separation is completed. If the hepatic veins have two or three branches, both of the final two vessels and the part of inferior vena cava valve where two vessels join should be retained (Fig. 34.1).

Fig. 34.1
Overhang left hepatic vein to determine the suspension and splitting line
When dissecting the hepatic hilus, the round ligament roots should be cut first, followed by separation of the left branch of the hepatic artery, the left portal vein, and the left hepatic duct. The total length of the left branch of the hepatic artery should be completely exposed, taking care to retain the four initial segments of the hepatic artery segment. If the origin of the fourth segmental arteries in the left branch of the hepatic artery is very high and relatively thick, the hepatic artery needs to be recut to fit the gastroduodenal artery stump. When the bile duct and artery are difficult to identify, injection of methylene blue can help to confirm their location. Ligate the fourth paragraph of the portal vein branch and free it, separating it along the right side of the navel. Then, the entire left portal vein can be completely separated (Fig. 34.2).