Fig. 9.1
The anterograde Spirus EndoEase Discovery SB overtube utilized primarily for anterograde deep enteroscopy. The same overtube can be used for retrograde procedures but only with a small bowel enteroscope. Reprinted with permission of Spirus Medical LLC
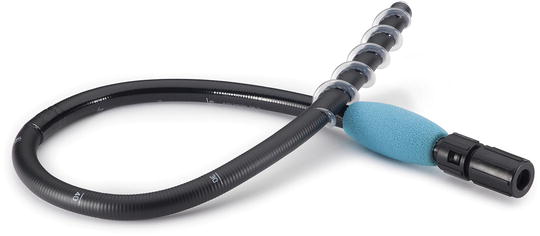
Fig. 9.2
The retrograde Spirus EndoEase Vista overtube is shorter and wider than its anterograde counterpart. It can be used with all small bowel enteroscopes as well as some pediatric colonoscopes with diameter <11 mm. Reprinted with permission of Spirus Medical LLC
Two operators are usually required to perform the spiral enteroscopy technique given the fact that both the overtube and the instrument have to be manipulated during the procedure. The overtube is installed on the enteroscope using an interlocking device, which can switch between a longitudinal (advance-withdrawal) and rotational axis of freedom for the scope within the overtube. The procedure can be performed with moderate sedation, monitored anesthesia care (deep sedation) or with general anesthesia depending on patient, indication and operator variables. If general anesthesia is used for anterograde procedures, it is advisable to deflate the endotracheal balloon while the spiral is advanced through the upper esophagus to avoid trauma. Infrequently, in patients with significant cervical spine disease or cervical osteophytes, the overtube cannot be advanced past the upper esophagus and an alternative enteroscopy method has to be employed [2]. Once past the upper esophageal sphincter, the fixed overtube-enteroscope unit is carefully advanced through steady rotation through the stomach into the duodenum, keeping in mind the possibility of occult strictures. Non-obstructive esophageal Shatzki’s rings are usually inconsequential, but strictures less than 15 mm in diameter should be traversed with caution. Once the overtube engages the pylorus and duodenum, the scope-overtube unit usually advances fairly easily with steady clockwise rotation into the small bowel. When the entire effective part of the overtube has been inserted in the patient and rotational advancement stops or when the operator encounters unusual rotational resistance, the unit can be unlocked again and the scope advanced independently in the small bowel to the maximal point of insertion or until pathology is found.
For the retrograde approach, the overtube serves primarily to “splint” the endoscope (usually an enteroscope) during insertion into the colon and avoid looping (Fig. 9.3). The retrograde spiral overtube can rarely be engaged through the ileocecal valve. Instead, in a relatively straight configuration and under favorable valve orientation, the enteroscope itself can be advanced relatively easily in the ileum (Video 9.1). In a small study, the terminal ileum was intubated in 100 % of patients and the depth of insertion past the ileocecal valve was estimated at 100 cm (range 50–150 cm) [3]. Controlled visualization and, on a case-by-case basis, endoscopic therapy occurs during system withdrawal, which is essentially the reverse of the process described previously (i.e., counter-clockwise rotation of the overtube with the scope either in “locked” or “free” position to allow a more nimble handling of the scope as well as diagnostic and therapeutic interventions). In order to increase the traction of the overtube on the small bowel, only the minimum amount of gas (air or CO2) is insufflated during advancement. A more detailed description of the procedure is available [1].


Fig. 9.3
The retrograde Spirus Vista overtube assembled on a 250 cm enteroscope. Note that the distal 20 cm of the scope are extending outside of the overtube during insertion to allow mobility of the scope and avoid excessive tension on the bowel wall. Some endoscopists prefer to have the overtube withdrawn all the way to the scope hub when they introduce the instrument through the rectum. After the scope is introduced for at least 60–70 cm, the colon loops are straightened and the overtube is advanced through the anus by gentle rotation while the colon lumen is kept in the field of view of the scope. Reprinted with permission of Spirus Medical LLC
Technical Success
The technical success rate defined as the ability of the instrument to advance past the proximal jejunum in patients with normal anatomy is approximately 95 % [2]. The most common reasons for failure are luminal strictures, abnormal or unusual anatomy (J-shaped stomach or narrow duodenal sweep) and anesthesia instability [2]. The depth of insertion is on average 200–250 cm post-pyloric (range 10–600) and likely corresponds to the limit between the distal jejunum and proximal ileum, although these measurements have not been adequately validated [2, 4–6]. No clear predictors of the depth of insertion have been identified, although this is an important aspect of these procedures [7]. The average time to reach the maximum depth of insertion is variable but in general it is shorter than either single- or double-balloon enteroscopy [8–10]. Akerman et al. reported an average insertion time of 18.7 min and total procedure time of 29 min [4]. In the US multicenter trial, the maximal extent was reached in an average of 22.1 ± 11.5 min, whereas the mean total procedure time for diagnostic studies was 34.4 ± 10.1 min and 11.4 min longer (range 0–73 min) for therapeutic procedures [2]. However, the depth of insertion and the rate of complete or pan-enteroscopy achieved with spiral enteroscopy appears to be inferior to that of double-balloon enteroscopy (DBE). In a small study using a combined anterograde and retrograde approach, pan-enteroscopy was accomplished in only 8 % of patients using spiral enteroscopy versus 92 % with DBE [10]. The learning curve with this system seems to be relatively quick. A selected group of experienced gastroenterologists were able to acquire the skills for spiral enteroscopy with fewer than 10 procedures in a dedicated training environment [11].
Diagnostic and Therapeutic Yield
The diagnostic and therapeutic yield of spiral enteroscopy in non-IBD patients is similar to other device-assisted deep enteroscopy techniques. Significant small bowel abnormalities are found in 33–75 % of symptomatic patients [2, 8, 9, 12]. Selecting patients via preliminary non-invasive studies such as capsule endoscopy increases the yield [2, 6, 8]. Diagnostic and therapeutic interventions can be performed in over 70 % of patients with positive findings [2, 8]. One potential advantage of spiral enteroscopy over other methods is that the endoscope can be withdrawn completely from the patient while maintaining the overtube in a stable position, thus allowing repetitive maneuvers such as piecemeal polypectomy or foreign body retrieval.
Comparison with Other Deep Enteroscopy Techniques
Several small studies compared the technical performance and diagnostic yield of spiral enteroscopy with double-balloon (DBE) or single-balloon enteroscopy (SBE) [8, 10, 13, 14]. The only randomized trial found that the depth of insertion and the ability to perform bi-directional panenteroscopy (combining oral and anal approach) was significantly higher with DBE compared to spiral enteroscopy (92 % versus 8 %, p = 0.002) but at the expense of a longer procedure duration. However, the diagnostic and therapeutic yields were similar [10]. In contrast, a multi-center larger prospective cohort study found no difference in insertion depth, procedure duration, and diagnostic and therapeutic yields between the two techniques. Panenteroscopy was not attempted in this study and, as mentioned earlier, the depth of insertion is very subjective, technique-dependent and difficult to validate [8]. In a retrospective single-center study, the average depth of maximal insertion was found to be higher with spiral enteroscopy than SBE (301 cm versus 222 cm, p < 0.001) but procedure duration and diagnostic yield were not significantly different, although there was a trend for longer procedure time with SBE [9]. A comparison of the three most popular deep enteroscopy modalities is provided in Table 9.1.
Table 9.1




Performance comparison of the three most popular deep enteroscopy techniques
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








