Fig. 14.1
Endoscopic appearance of terminal ileal CD with deep ulceration
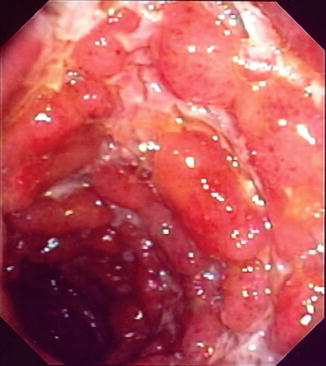
Fig. 14.2
Endoscopic appearance of colonic CD demonstrating the characteristic “cobblestone” appearance with a combination of deep ulcers and inflamed, edematous mucosa
Endoscopy together with other diagnostic modalities can differentiate CD from UC in more than 85 % of patients [23]. In a prospective study of more than 350 patients with IBD followed up for more than 22 months, index colonoscopy and biopsy were accurate in distinguishing CD from UC in 89 % of cases. IBD diagnosis was revised in 4 % of cases, and the diagnosis of indeterminate colitis remained in 7 % of cases [24].
Endoscopic Scoring Indices Used to Evaluate Crohn’s Disease
The Crohn’s Disease Endoscopic Index of Severity
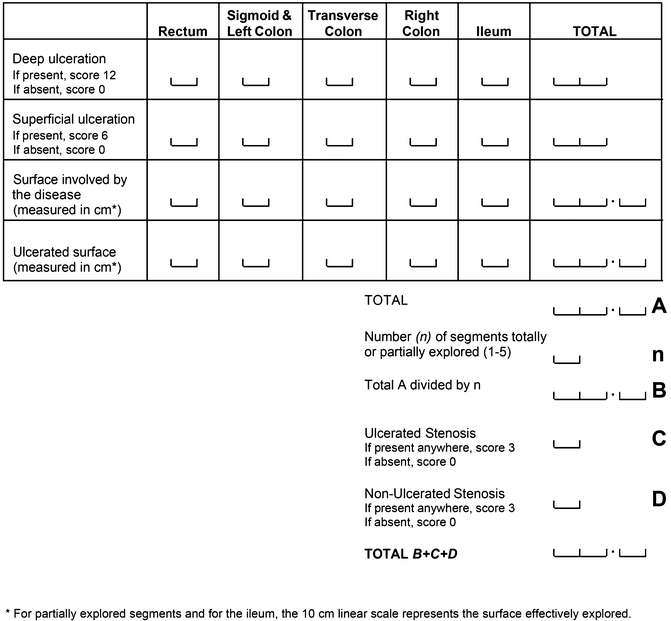
Index Development, Validation and Utilization
The Crohn’s Disease Endoscopic Index of Severity (CDEIS) is the longest standing of the endoscopic indices used for evaluating endoscopic disease activity [10]. It was developed by the GETAID (Groupe d’Etude Thérapeutique des Affections Inflammatoires Digestives) group in 1989 as part of a multi-phase study. In the first phase of developing the index, two endoscopists (one of whom performed the procedure) scored 5 ileocolonic segments (rectum; sigmoid and left colon; transverse colon; right colon; and ileum). In each of these segments data on nine mucosal lesions (pseudopolyp, healed ulceration, frank erythema, frankly swollen mucosa, aphthoid ulceration, superficial or shallow ulceration, deep ulceration, non-ulcerated stenosis, and ulcerated stenosis) was collected along with the percentage of mucosal surface with disease involvement and the percentage with ulceration, indicated by the endoscopists on a 10 cm visual analogue scale. By dividing the number of segments with endoscopic lesions by the total number of segments explored, a score was also determined for individual segmental rectocolonic frequency (ISRCF). Using multiple linear regression techniques, the CDEIS was derived by correlation between these lesions and an endoscopist’s global evaluation of lesion severity (GELS) (also determined using a 100 mm visual analogue scale).
A final score with four lesions (superficial or shallow ulceration, deep ulceration, non-ulcerated stenosis, and ulcerated stenosis) along with estimates of extent involvement were weighted to create the final score with a range of 0–44 (Fig. 14.3).
In each bowel segment, superficial and deep ulcerations are given a score of 6 and 12 points, respectively. These are added to an estimate of diseased surface and an estimate of ulcerated surface (expressed on a 100 mm visual analogue scale). These scores are summed and the total is divided by the number of bowel segments examined (1–5). An additional 3 points are given if a non-ulcerated stenosis is present and further 3 should be added if an ulcerated-stenosis is seen (i.e., 6 points if both are present). The sum of these variables provides the final CDEIS. Clearly, absent and inaccessible segments (due to impassable stenosis or technical difficulties) are not accounted for in these calculations.
As part of the original study the CDEIS was partially validated and its operating characteristics, with regards to criterion validity, inter-observer agreement and responsiveness were demonstrated. Very good correlation with lesion severity and minimal intra-observer variability suggested the index was representative of disease activity and was also reproducible (though the independence of the endoscopists was limited by them being present in the same procedure room).
Responsiveness was subsequently assessed during the second phase of the study in a trial of prednisolone in a group of 54 patients with active CD. These patients were separate from the group used in the first phase. A strong correlation between changes in the CDEIS and GELS was demonstrated when scores for disease activity at baseline and after 3–5 weeks of treatment were compared.
In a recent study, the properties of the CDEIS were re-assessed amongst a group of four expert, central readers. Fifty recorded procedures were scored three times in random order. Results demonstrated “substantial” to “almost perfect” intra- and inter- observer reliability [25].
Endpoints, Definitions and Cut-off Values
The GETAID group followed-up their pioneering work and continued to investigate the properties of the CDEIS in a prospective, multicenter study of 142 patients with moderately-to-severely active CD [5]. Patients were treated with prednisolone (1 mg/kg/day) and underwent serial endoscopies at baseline and after 3–7 weeks of treatment. In this study remission was defined as: (1) no lesions, (2) only scarred lesions, or (3) minor lesions with at least a two-grade decrease on a five-degree scale of endoscopic severity (grade 1: no lesions to grade 5: very severe) with no residual deep ulceration. Though no numerical cut-off points were defined for remission or response in that study, subsequent collation of data from 5 prospective GETAID studies has made this possible [26]. From a total of 562 colonoscopies performed in 231 patients with active CD the following definitions were suggested:
Complete remission (complete mucosal healing)—CDEIS <3
Remission—CDEIS <6
Response—a decrease in CDEIS by >5
These cut-off values were used in the recent MUSIC trial (Endoscopic MUcoSal Improvement in Patients with Active Crohn’s Disease Treated with Certolizumab Pegol) when investigating mucosal healing with certolizumab pegol (a pegylated monoclonal antibody fragment to tumor necrosis factor alpha) therapy [15]. This study used the absolute change in CDEIS at 10 weeks as the primary end point with endoscopic response, endoscopic remission and complete endoscopic remission as secondary endpoints. However, these definitions have not been consistently applied in other trials. For example, a 2011 study to investigate the mucosal healing effect of methotrexate in CD used a score of <4 (rather than <3) to define complete mucosal healing [27]. Cut-off values to stratify disease activity into mild, moderate and severe have also been suggested. During the ACCENT 1 trials (A Crohn’s Disease Clinical Trial Evaluating Infliximab in a New Long-term Treatment Program) these thresholds were arbitrarily defined as <5, 5–15 and >15 respectively [28].
Until recently, no formal analyses had been carried out to investigate the importance of minimal clinical change in CDEIS. However, in a recent study Ferrante and colleagues attempted to assess the minimal necessary improvement in endoscopic activity that could serve to define endoscopic response and that could predict sustained clinical benefit [29]. To do this they undertook a post-hoc analysis of data from the SONIC trial (Study of Biologic and Immunomodulator Naïve patients in Crohn’s Disease) [14]. The authors studied several predefined endpoints that have been previously used as outcome measures in clinical trials. They studied different values for absolute reduction in CDEIS (by at least 3 [30], 4 [26] or 5 [14, 26] points) as well as relative reduction (by at least 50 % [31] or 75 % [13]). Their results suggested that defining endoscopic response as a reduction in CDEIS from baseline of at least 50 % provided an endpoint at week 26, which was predictive of corticosteroid-free remission at week 54. Using a relative, rather than an absolute, decrease has the additional benefit that it accounts for patients with relatively low baseline endoscopic disease activity who cannot achieve large reductions in absolute values. This definition for endoscopic response shows significant potential as a reliable, achievable and meaningful endpoint. However, the authors conclude that prospective trials are necessary to validate the mid-term (1 year) outcomes described as well as to investigate effect on long-term (>3 years) disease-modifying outcomes.
The CDEIS has certain limitations and is somewhat complex to use. Assessments made at endoscopy require conversion from a visual analogue scale to calculate the final score and making accurate assessments requires training as well as experience. Its usefulness in day-to-day practice therefore appears limited. Another limitation is that the final score does not reflect the number of affected segments, the location of stenoses (if present) or the severity per segment. Studies investigating the correlation of the CDEIS with clinical disease activity scores—most commonly the Crohn’s Disease Activity Index (CDAI)—have yielded conflicting results. Some studies had suggested a good correlation [32] between the two, whereas others (including the original CDEIS studies) demonstrated the opposite [5, 10, 11, 33]. The apparent poor correlation demonstrated in some of these studies could be explained in several ways. Relatively limited lesions (e.g., proctitis) can cause severe symptoms, whereas patients with diffuse but rather superficial ulceration can present with rather mild symptoms. A single stenosis without further ulcerations can be the cause of severe abdominal pain and a high clinical disease index. Finally, the mucosal appearance may not accurately reflect systemic manifestations of active inflammatory disease. It is also possible that in the case of CD affecting the small bowel proximal to the portion of the distal ileum visualized at ileocolonoscopy, the CDEIS could underestimate the degree and extent of disease activity.
The Simple Endoscopic Score in Crohn’s Disease
Index Development, Validation and Utilization
The Simple Endoscopic Score in Crohn’s Disease (SES-CD) was devised in 2004 to offer a less complex and more user-friendly alternative to the CDEIS. Daperno and colleagues began by incorporating items from the CDEIS with high interobserver agreement into their novel index [34]. The SES-CD grades four items: ulcer size (diameter 0.1–0.5 cm, 0.5–2 cm, or >2 cm); proportion of ulcerated surface (<10 %, 10–30 %, or >30); proportion of the surface area affected by any disease lesion (<50 %, 50–75 %, or >75 %); and stenosis (single, multiple, impassable with a colonoscope). Each item is scored from 0 to 3 in each of the five ileocolonic segments (as described in the CDEIS: rectum, sigmoid and left colon, transverse colon, right colon, and ileum). As part of their regression modeling analysis it was found that the sum of the scores for the four segments should undergo a relatively minor arithmetic manipulation to give the optimal score. However, for the sake of simplicity the sum of the values for the four variables, for the five bowel segments was decided upon as the final score giving a range of 0–60, with higher scores indicating more severe disease (Table 14.1). Apart from its relative simplicity, another proposed advantage of the SES-CD above CDEIS is its emphasis on ulceration as the most likely lesion to change with therapy [35].
SES-CD values | ||||
|---|---|---|---|---|
Variable | 0 | 1 | 2 | 3 |
Ulcers | None | Aphthous ulcers (Diameter 0.1–0.5 cm) | Large ulcers (Diameter 0.5–2 cm) | Very large ulcers (Diameter >2 cm) |
Ulcerated surface | None | <10 % | 10–30 % | >30 % |
Affected surface | Unaffected segment | <50 % | 70–75 % | >75 % |
Stenosis | None | Single, can be passed | Multiple, can be passed | Cannot be passed |
As part of the process of developing the SES-CD, the authors also investigated its operating characteristics. Agreement for each of the items modified from the CDEIS was studied in a series of 71 procedures. Two endoscopists (both present in the procedure room but not communicating with each other) graded each item from the SES-CD. This exercise successfully demonstrated high intra-observer agreement.
The construct validity of the SES-CD was demonstrated by correlation with the CDEIS. The authors of the original paper demonstrated a strong correlation between their simplified index and its more complex alternative. This finding has been subsequently confirmed in a Finnish cross-sectional study of 86 patients with CD undergoing ileocolonoscopy. Near perfect correlation between the two scores was demonstrated when procedures were examined by a single endoscopist [36]. Near perfect correlation was also seen in a recent study where four expert central readers each graded 50 recorded procedures on three occasions [25]. This study not only demonstrated the close relationship between the two scores and reliability of central readership but also added validity to both scores by reporting a substantial correlation with an endoscopist’s global rating of disease (based on a visual analogue scale).
The responsiveness of the SES-CD has also been studied in a number of ways. By performing sub-group analysis on data from the SONIC trial, Ferrante et al. [29] demonstrated an excellent correlation between changes in SES-CD and CDEIS values at week 26. In a smaller, prospective cohort study carried out as part of the Finnish study described previously [36], 32 patients underwent a follow-up endoscopy at an average of 4 months from baseline. This also demonstrated that changes in SES-CD and CDEIS between these two examinations correlated highly.
Correlation between the SES-CD and clinical parameters has also been investigated. While the SES-CD was shown to have only a weak correlation with the CDAI in the study that originally described the simplified score [34], a subsequent trial demonstrated a moderate correlation [36]. However, the changes over time seen in these indices correlate poorly. The same pattern was described for the relationship of SES-CD with C-reactive protein (CRP).
Endpoints, Definitions and Cut-off Values
As with the CDEIS, clearly defined and validated endpoints and cut-off values for disease severity using the SES-CD have not been described. However, through a combination of expert consensus [37] and prior trial experience, boundaries for disease activity have been arbitrarily set. The following cut-off values are generally accepted and have been used as endpoints in clinical trials [37, 38]:
0–2 Remission
3–6 Mild inflammation
7–16 Moderate inflammation
>16 Severe inflammation
As is seen with cut-off values to describe severity using the CDEIS various, minor alterations have been suggested [39] and used as boundaries for the SES-CD. For example, in a trial comparing the two scores, an SES-CD of >15 was used to define severe disease [36].
The minimal clinically important change in SES-CD values was investigated during post-hoc analysis of data [29] from the SONIC trial (described in the CDEIS section). Though values for absolute change in the SES-CD were not studied in detail (as was undertaken for the CDEIS) the authors demonstrated that a relative reduction of 50 % from baseline score at the week 26 endoscopy accurately predicted corticosteroid free remission at week 50. Another post-hoc analysis study [40], this time examining data from the EXTEND trial (EXTend the Safety and Efficacy of Adalimumab Through ENDoscopic Healing), showed that for CD patients treated with adalimumab, an SES-CD score of 5 measured at week 12 represented the optimal dichotomizing points for predicting clinical remission at week 52.
Owing to its relative simplicity and demonstrated high degree of correlation with CDEIS, the SES-CD has gained favor. It is now in frequent use as an entry criteria [41] and as endpoints [9, 27, 42, 43] in clinical trials. Elsewhere the two scores have been scored alongside one another [44]. However, this strategy may become unnecessary as data demonstrating correlation and experience interpreting the simplified score grows. The SES-CD is also eminently more feasible for application to daily practice than the CDEIS, with some experienced endoscopists advocating its routine use in every ileocolonoscopy where CD is assessed [36]. However, by virtue of the way the ileocolonic segments are divided it is possible that both scores have the potential to overestimate colonic disease or mild changes seen in several segments. Conversely, ileal disease alone, or a limited but severe disease may be underestimated by both systems [1].
Rutgeerts Score
Index Development, Validation and Utilization
The Rutgeerts score is the long-standing and widely accepted scoring system for the assessment of Crohn’s disease activity in the postoperative setting [45, 46]. Since its development more than two decades ago and despite limited validation it has been the gold standard and no other scoring systems are in common use for this purpose [47]. It is used to describe the severity of endoscopic changes (recurrence of disease) seen at the ileocolic anastomosis and in the preanastomotic ileum after ileal or ileocolic resection. The authors devised their score based on the observation that endoscopic recurrence, particularly at the anastomosis, precedes clinical recurrence [48]. Moreover, it was noted that the severity of the endoscopic changes seen correlated with the likelihood of subsequent clinical relapse. Lesions seen at endoscopy are graded on a five-degree scale (i0–i4):
i0 No lesions
i1 Fewer than five aphthous lesions
i2 More than five aphthous lesions with normal mucosa in between or skip areas of larger lesions, or lesions confined to ileocolonic anastomosis (that is, <1 cm in length)
i3 Diffuse aphthous ileitis with diffusely inflamed mucosa
i4 Diffuse inflammation with already larger ulcers, nodules, and/or narrowing
Endpoints, Definitions and Cut-off Values
When applied to an asymptomatic patient in the year following surgery this index not only provides a real-time assessment of endoscopic recurrence but allows for accurate stratification of patients into groups at high or low risk of developing symptoms. It therefore has significant prognostic value: 80–85 % of patients with a score of i0 or i1 (Video 14.3) will be asymptomatic 3 years after surgery compared with fewer than 10 % of those with a score of i3 or i4 (Video 14.4) [49, 50]. As well as predicting the future development of symptoms, estimates of progression of mucosal changes can be made. In Rutgeerts’ original study of 89 patients having undergone ileal resection for CD, 80 % of patients with i0 or i1 lesions at the postoperative endoscopy had unchanged lesions at 3 years. However, mucosal disease progression was noted in 92 % of patients with i3 or i4 lesions. Though this property was validated in the original work, the reproducibility of the score has not been fully and prospectively validated. Nonetheless, it has been extensively used for clinical trials [51–53] and integrated into many postoperative algorithms (Fig. 14.4). For example, the authors of this chapter advocate planning an ilecolonoscopy at 6 months to 1 year after ileocolic resection (assuming patients remain asymptomatic). The Rutgeerts’ score measured at this procedure, along with clinical, biochemical and imaging (if appropriate) parameters should be used to guide therapeutic decisions. Based on the evidence previously described, there is good rationale that those with i3 and i4 lesions should undergo treatment intensification, even in the absence of symptoms. Equally, for those on no treatment with i0 and i1 lesions, monitoring for the recurrence of symptoms alone is advocated. If treatment had been continued after surgery, there is no clear evidence whether this should remain unchanged or withdrawal should be considered. Assuming an ongoing asymptomatic course, then endoscopic re-evaluation at 3 years post surgery is suggested. The duration of this interval is, in part, arbitrary but also draws on evidence from the aforementioned studies.
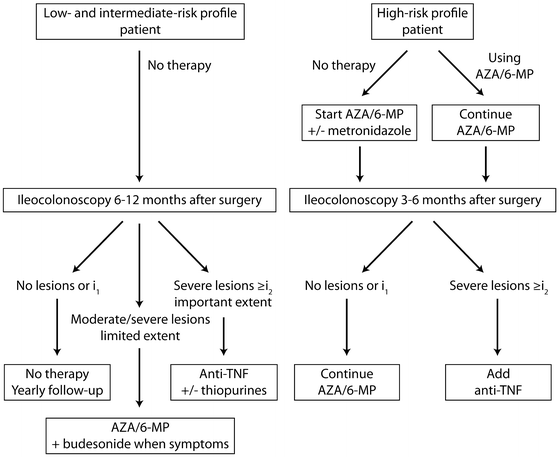

Fig. 14.4
The use of Rutgeerts score in a postoperative surveillance and treatment algorithm for CD. AZA = azathioprine, 6-MP = 6-Mercaptopurine. (See Fig. 12.1) Reprinted with permission of S. Karger AG, Basel from van Lent AU, D’Haens GR. Management of postoperative recurrence of Crohn’s disease. Dig Dis. 2013;31(2):222–8
A grade of i2 on the Rutgeerts score predicts an intermediate risk of clinical recurrence of disease [35]. Prognosis for this group is more difficult to define than for the other grades and is further complicated by its description, which is more subjective than the alternatives [16]. This has therefore divided opinion in its interpretation when defining disease recurrence for clinical trials. Some studies have defined postoperative recurrence as a score of i2 or above [51], whereas others have opted for i3 and above [54]. Others have even included those with i1 lesions in their recurrence group [55], though evidence would suggest that this is an unnecessarily stringent definition. In an attempt to overcome the uncertainly surrounding this issue some authors have modified the Rutgeerts score to divide the i2 grade into two groups: i2a and i2b. For the purpose of their study investigating recurrence rates in patients treated with azathioprine versus those on mesalazine, Reinisch and colleagues did this [56]: They defined i2a as “moderate endoscopic recurrence,” demonstrated by >5 aphthous lesions with normal mucosa between the lesions, or skip areas of larger lesions. In turn, i2b lesions were defined as those confined to the ileocolonic anastomosis (i.e., 1 cm long). Similar dissonance exists in day-to-day practice with some clinicians preferring to recommence treatment based on i2 images and others taking a more conservative approach. Currently insufficient evidence exists to justify treatment on this basis alone and the authors of this chapter advocate a strategy based on close clinical observation.
These examples serve to demonstrate that further work needs to be carried out to accurately define the postoperative risk of symptomatic recurrence in this group of patients. Such studies are already in progress and this well-established index is likely to undergo further attempts at modification in the near future.
The Definition of Mucosal Healing and Endoscopic Response in Crohn’s Disease
Although the characteristic appearances of CD at ileocolonoscopy are well characterized, there is no validated definition of mucosal healing [57]. Endoscopic remission is an alternative, synonymous term sometimes used to describe healed mucosa but is equally without validated definition. A simplistic and perhaps most intuitive definition of mucosal healing relies on absence of any mucosal ulceration. This definition has the clear advantages of its relative ease of use and its property of dividing patients in a binary fashion into those with residual ulceration and those without. Although this definition has been used in designing endpoints for clinical trials, it is less useful in clinical practice where a spectrum of disease needs to be appreciated in context with many other parameters (symptoms, abdominal imaging, serum and fecal biomarkers, for example).
In fact, even within clinical trials, evidence is gathering that such a stringent definition for mucosal healing is less optimal than first imagined. For example, the use of this definition would classify a patient in whom multiple, deep ulceration had improved leaving only a solitary small ulcer as a “non-responder.” The difficulty in achieving this definition can be demonstrated using data from the randomized, placebo-controlled EXTEND trial, where mucosal healing was used as a primary endpoint for the first time when studying a biological agent [13]. In this study, mucosal healing was assessed by a blinded central reader and any disagreements between sites and central readers were adjudicated by up to two additional central readers. Mucosal healing was defined as absence of ulceration in patients with ulceration at baseline and a secondary endpoint of reduction in CDEIS by >75 % was also set. Of the 62 patients with CD given adalimumab, 15 (24 %) achieved mucosal healing following the induction phase and the same number maintained this at 52 weeks. Based on this observation, complete absence of ulceration is now often considered by many experts to be too difficult to achieve when assessing drug efficacy in a clinical trial or in daily practice. Though evidence would suggest that achieving mucosal healing is associated with favorable outcomes [58, 59] it remains unclear exactly what degree of healing is required to achieve these long-term clinical benefits.
Recent investigation has attempted to define a more achievable and pragmatic definition for mucosal healing by studying the minimal clinically important improvement in endoscopic disease. Though the SONIC trial [14] used complete absence of ulceration as a secondary endpoint when studying mono- versus combo-therapy (using azathioprine and infliximab, in immunomodulator naïve patients with CD), post-hoc analysis of results [29] led to the conclusion that a reduction in the inflammatory score (as measured by the SES-CD or CDEIS, both discussed in subsequent sections of this chapter) of 50 % at week 26 compared with baseline score was the minimal endoscopic improvement associated with clinical benefit (Video 14.5). The authors described this degree of change as “endoscopic response”—an endpoint that showed good predictive value for maintaining clinical benefit at week 50 using a variety of clinical endpoints. However, it is not yet known whether the mid-term improvement in clinical outcome associated with endoscopic response in this study will translate into disease-modifying, long-term benefits. Though validation of this endpoint and investigation of long-term outcomes will require further prospective studies, this target has significant potential for trials as well as implications for targets in clinical practice. For example, integrating endoscopic re-assessment at 26 weeks in treatment algorithms may identify patients who would benefit from early treatment intensification.
The previously described evidence demonstrates how using the presence or absence of ulceration alone lacks the sensitivity to describe grades of activity or partial endoscopic responses. Its responsiveness as an evaluative index for dose finding or early phase trials seeking a signal of efficacy is also unknown. To address these shortcomings, endoscopic indices, which allow objective quantifiable assessments, appear useful. An ideal index should detect meaningful changes in mucosal appearance with treatments of known efficacy (responsiveness) and remain unchanged in static disease (reliability). As part of their validation, indices should have limited inter- and intra-observer variability. These indices, along with their development and definitions will be considered in the next section of this chapter.
The focus of this chapter concerns the use of endoscopy to follow the clinical course of CD and this section has, therefore, discussed only endoscopic definitions of mucosal healing. There are, of course, other ways of defining mucosal healing, including the use of histological resolution of abnormalities or examination of mucosa using confocal laser endomicroscopy. These techniques are considered beyond the scope of this chapter but are discussed in the relevant chapters of this book.
The Clinical Relevance of Mucosal Healing in Crohn’s Disease
The Relationship between Clinical and Endoscopic Disease Activity
The lack of correlation between clinical measures of disease activity (using the CDAI) and endoscopic assessments for CD has been demonstrated on several occasions [10, 11]. In a study to investigate the relationships between disease activity and serum and fecal biomarkers in patients with CD, no correlation was observed between the CDAI and SES-CD [60]. It is also worth noting that though serum (CRP and interleukin-6 concentrations) and fecal biomarkers showed the same poor correlation with CDAI, they both correlated strongly with SES-CD values. The correlation between symptoms and endoscopic scores is known to be particularly weak when attempting to predict the degree of endoscopic activity present based on clinical scores alone [5]. An example of this was demonstrated in analysis of data from the SONIC trial [14], comparing infliximab or azathioprine monotherapy with a combination of the two drugs. Inclusion to the trial required a CDAI score of >220, suggesting moderate disease at least, but findings demonstrated that 18 % of patients meeting this criteria had no objective evidence of endoscopic disease activity. This disparity has clear implications for research studies (higher placebo rates, lower estimated effect size) as well as decision-making in clinical practice. It is possible that these findings reveal the limitations [47, 61] of the CDAI as an instrument for assessing clinical activity, though alternative explanations do exist, as outlined previously.
The poor predictive value of clinical disease scores when estimating endoscopic disease activity is even more pronounced in the postoperative patient. As discussed in the section describing the Rutgeerts score for postoperative disease recurrence, mucosal changes are usually seen before patients develop symptoms [45, 48]. Along with the limitations of the CDAI considered above, this well-understood pattern of chronology means that the CDAI does not have the appropriate operating characteristics to use in this setting. This was demonstrated in a study of 110 postoperative patients, of which CDAI values could correctly predict endoscopic recurrence in only 65 % [62]. This finding has been confirmed in other, similar studies [63], one of which showed that using a CDAI cut-off of 150 resulted in a sensitivity of only 70 % (though specificity was better at 81 %[64]). Owing to these findings, routine endoscopic revaluation at 6–12 months following surgery remains the gold standard for diagnosing postoperative recurrence of disease. This proactive strategy is essential to guide management aimed at preventing symptomatic recurrence in patients with endoscopic disease activity.
The Impact of Endoscopic Disease Activity on Outcomes
The association between endoscopic disease activity, however defined, and clinical outcomes is surely the key relationship when considering how to monitor patients with CD based on mucosal assessment. It also forms the driver behind the increasing use of endoscopic outcome measures in trials investigating novel therapies. The natural history of CD is a progression from an inflammation predominant disease (90 % at presentation [35]) to one characterized by structuring and penetrating complications [65]. Surgery is required in the majority (80 % at some point in the disease course) and is not curative, with rates of repeat surgery as high as 70 % in some series [66]. Strategies aimed at the amelioration of symptoms without necessarily demonstrating mucosal improvement have been ineffective at significantly altering this pattern [3]. The advent of biological therapies with the property to heal mucosa renewed hope that better outcomes for patients with CD was possible. The dramatic effect on endoscopic appearances observed led to the prediction that directly targeting tissue damage at the mucosal level could result in rapid and sustained mucosal restitution. It was hoped that this in turn should halt the progression of disease and development of complications. However, to justify the additional resources needed and inconvenience to patients involved in a follow-up strategy based on endoscopic results, evidence was needed to support this prediction.
Following studies carried out in the 1990s demonstrating clearly that mucosal healing was achievable with infliximab [7] the relationship between deep mucosal ulceration and long-term clinical outcomes was more clearly defined. Allez and colleagues revealed that CD patients with deep and extensive (involving at least 10 % of a colonic segment) ulceration at index ileocolonoscopy had a significantly higher rate of colectomy over subsequent years than those without [67]. In their longitudinal series, 62 % of those with deep and extensive ulcers underwent colectomy compared to 18 % without. They concluded that those with severely diseased mucosa had a more aggressive disease course with increased rates of penetrating complications and surgery. The findings of these two fundamental pieces of work taken in conjunction suggested that being able to control disease activity at the mucosal level could indeed deliver beneficial outcomes in CD.
Since the aforementioned studies, evidence has continued to emerge to support the notion that setting and reaching endoscopic targets improves both short- and long-term clinical outcomes. Data from the ACCENT 1 study [12] along with its endoscopic sub-study [32] provided good examples of this principle. Results from the primary trial showed that achieving mucosal healing was associated with a more durable clinical remission. Those reaching this endpoint at week 54 had a significantly longer time to relapse (median 19–20 weeks) than those who did not (4 weeks). In the sub-study, a reduction in hospitalization rates amongst those achieving mucosal healing was also demonstrated. No patient with mucosal healing at both time points (10 and 54 weeks) required hospital admission. This was compared to 4/16 (25 %) of those with healing at one time point only and 34/74 (46 %) of those without healing. These findings have been supported by a large cohort studies. One of these, carried out in Leuven [58], included 183 patients who responded to induction therapy with infliximab and were followed up over a median of almost 6 years while on maintenance treatment. Amongst this cohort, lower rates of hospitalization were again demonstrated along with reduced incidence of surgery in those achieving mucosal healing. Similar results were also generated in an even larger cohort study from the IBSEN (Inflammatory Bowel South-Eastern Norway) group including 227 newly diagnosed patients followed for a total of 8 years [8] in an era when biological treatment was not yet available. In addition to the above, this study also demonstrated that absence of mucosal ulceration at one year predicted a reduced need for steroids and decreased clinical disease activity over the follow-up period.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree