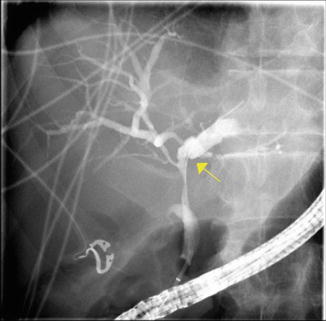
Fig. 22.1
(a) Marked intrahepatic right ductal stenosis and a tightly strictured left system filled with stones. (b) Patient was dilated with a 6 mm balloon. (c) Stone extraction. (d) Attempts to dilate the minute right system with a 6 Fr catheter was associated with a local extravasation. (e) The duct disruption was stented with a 3 Fr by 10 cm stent
Diagnosis of PSC
The discovery of PSC increasingly is based on the investigations of abnormal liver tests and incidental finding of intrahepatic biliary ductal dilatation on cross-sectional imaging as the majority (44–56 %) of the PSC patients are asymptomatic at the time of diagnosis [5, 6, 13]. A multicenter retrospective Italian study has found up to 17 % of asymptomatic PSC patients may have cirrhosis on liver biopsy at the time of diagnosis [6, 24].
Fatigue and pruritus are the initial presenting symptoms for symptomatic patients with PSC. The patients tend to develop jaundice, abdominal pain and weight loss with disease progression. Bacterial cholangitis is uncommon at presentation in the absence of dominant biliary stricture(s) or biliary intervention [6, 25].
ERCP and transhepatic cholangiography were once thought to be the reference standard for PSC diagnosis [26] before the era of magnetic resonance cholangiopancreatography (MRCP) [27]. The characteristic findings of cholangiography (Fig. 22.2) include short, multifocal, annular strictures alternating with normal or slightly dilated intervening segments called “beads on a string” [28]. A small case series (n = 10) has noted retraction of the major papilla into the duodenal wall in 70 % of the PSC patients (7 out of 10) with typical cholangiogram features [29]. In a recent prospective pilot study, endoscopic ultrasound (EUS) has also proved to be a valuable tool for accurately predicting extrahepatic disease in suspected PSC [30].
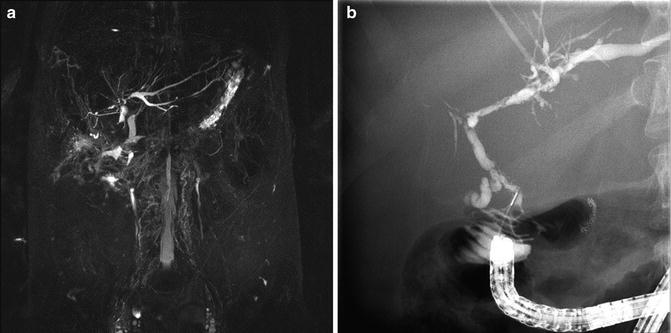

Fig. 22.2
(a) MRCP image and (b) ERCP image showing recurrent PSC in a patient after liver transplant
In the presence of typical cholangiogram findings, a routine liver biopsy is not required to confirm the diagnosis of PSC. However, a liver biopsy may be required to diagnose small duct PSC and suspected overlapping syndromes such as PSC with autoimmune hepatitis (AIH), and PSC with immunoglobulin G4 associated sclerosing cholangitis [25].
A wide range of auto-antibodies can be detected in the serum of patients with PSC (e.g., anti-neutrophil cytoplasmic antibody, anti-nuclear antibody, anti-smooth muscle, anti-endothelial cell antibody, anti-cardiolipin antibody, thyroperoxidase, thyroglobulin, rheumatoid factor). However, these antibodies have no routine role in the diagnosis of PSC [25].
Magnetic Resonance Cholangiography (MRCP) Versus ERCP
ERCP is an invasive procedure and can be associated with complications such as pancreatitis, cholangitis, bleeding, perforation (Fig. 22.3), and aspiration [27]. One large multicenter prospective study noted that among 942 diagnostic ERCPs performed there were 13 major complications (1.3 %) and 2 deaths (0.21 %). ERCP may be associated with post-procedural hospitalization in up to 10 % of patients [31]. In contrast to ERCP, MRCP is a non-invasive, complication-free technique, which has the advantages of not using contrast media or ionizing radiation and a relatively shorter time for the examination [32]. Blinded case control, comparative studies have shown, despite an overall better depiction of the biliary tree by endoscopic retrograde cholangiography (ERC), both ERC and magnetic resonance cholangiography (MRC) are comparable in diagnosing PSC [33, 34].
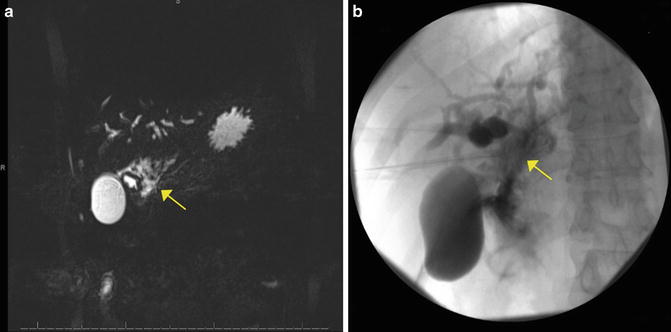

Fig. 22.3
(a) MRCP image and (b) ERCP image showing guidewire perforation at hilum in a patient with PSC
Endoscopic Therapy for Symptomatic PSC
With the improvement in the ability of MRCP in diagnosing PSC, the role of ERCP has changed from diagnostic to therapeutic intervention (Figs. 22.1, 22.4, Video 22.1). A large retrospective study from a tertiary center clinically followed 117 patients with PSC for a mean period of 8 years (range 2–20 years), of which 72 % (n = 84) of the patients with PSC required at least one therapeutic ERCP for symptomatic disease [19]. Of the 84 patients who underwent therapeutic interventions, 70 % (n = 59) had balloon dilation of biliary strictures, 51 % (n = 43) had stone extraction, and 51 % (n = 43) had biliary prosthesis placed to facilitate drainage of infected bile ducts and to improve the bile duct patency on one or more occasions. The overall complication rate was 7.2 % following therapeutic ERCP but there were no procedure-related deaths.
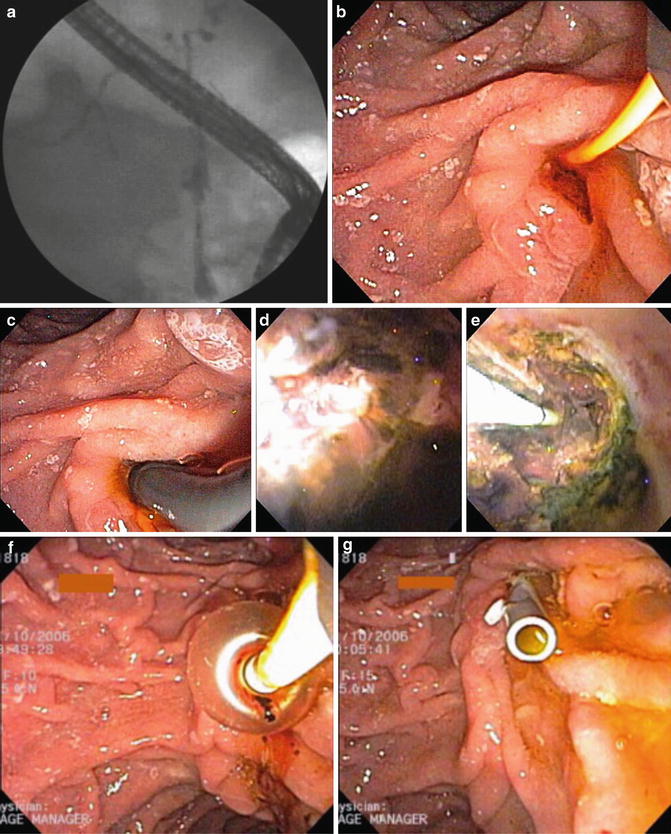

Fig. 22.4
(a) Cholangiography demonstrates high-grade extrahepatic and bifurcation strictures. (b) Following sphincterotomy, (c) a video cholangioscope is inserted to the bifurcation. (d) Note inflammatory change at the hilum and (e) common hepatic duct stone debris. (f) The latter is removed with balloon extraction followed by (g) stent placement
During the course of PSC, dominant (high grade) strictures (Fig. 22.1) may develop in approximately 36–56 % of the patients. These patients have increased risk for cholangiocarcinoma [13, 35, 36] (Fig. 22.5).
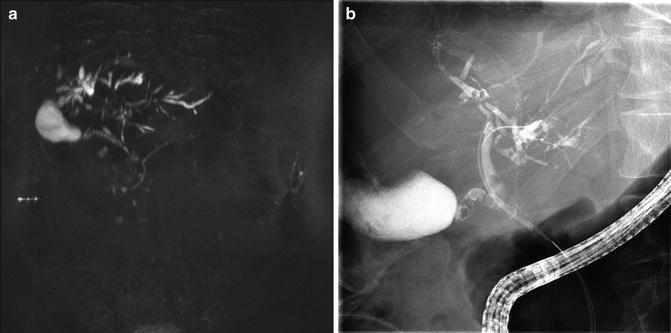

Fig. 22.5
(a) MRCP image and (b) ERCP image showing diffuse severe biliary strictures in a patient with PSC
Biochemical and clinical improvements have been reported with endoscopic therapy with stenting and/or balloon dilation of dominant strictures [36]. Moreover, there is some evidence to support that secondary liver fibrosis can be reversed by relieving biliary obstruction [37]. Finally, endoscopic therapy has been suggested to improve survival in patients with PSC. A retrospective study of 63 consecutive PSC patients, with a median follow-up of 34 months, noted that the observed survival rate over 5 years following endoscopic therapy (mostly balloon dilation of biliary strictures) was significantly higher than the predicted 5-year survival rate based on the Mayo clinic survival model (83 % vs. 65 %, p = 0.027) [38].
Several non-randomized studies have also noted PSC patients with dominant strictures benefiting from endoscopic intervention, including 81–94 % 5-year liver transplantation free survival rates [35, 38, 39]. Chapman and colleagues, in a large retrospective study, compared long-term outcomes (mean follow-up 9.8 years) of multiple endoscopic interventions (stent alone 46 %, dilation alone 20 %, both stent and dilation 17 %, failed interventions 17 %) in patients with dominant strictures (n = 80) and without dominant biliary strictures (n = 48). Patients with dominant strictures had more interventions (median of 3 [range 0–34]) compared to the patients without dominant strictures (median of 0 [range 0–7]; p <0.001). The major complication rate for ERCP was low at 1 %. Although repeat endoscopic therapies were found to be safe in this study, the overall survival was found to be worse for the patients with dominant strictures (mean survival 13.7 years) compared to the patients without dominant strictures (mean survival 23 years). Much of this survival difference was related to a 26 % risk of cholangiocarcinoma developing only in the patients with dominant strictures [36].
Predictors of Successful Outcome
Published series and case control studies have documented 53–76 % successful clinical outcomes of therapeutic ERCP in patients with PSC [40–43]. A large retrospective study (204 total ERCPs performed on n = 148 patients with PSC) noted clinical improvement in 70 % of patients with PSC following therapeutic ERCP (p = 0.0001). Of the patients with PSC, 53 % had resolution of their presenting complaints and maintained it at 3–6 months, which met the study criteria for clinical success. Endoscopic therapy (OR =4.23, 95 % CI 2.15–8.34) was found to be an independent predictor of the clinical success. Patients who had high bilirubin levels, dominant biliary strictures compared with those without (OR =3.73, 95 % CI 1.95–7.13), common bile duct strictures versus those who had strictures in other locations (OR =2.47, 95 % CI 1.27–4.81) were all more likely to have successful clinical and laboratory outcomes [44].
Complications of ERCP in PSC Versus Non-PSC
Endoscopic therapy for patients with PSC and dominant strictures has been undertaken for more than 20 years, but there are concerns about the risks versus anticipated benefits in instrumenting a sclerotic biliary tree. A large retrospective study (n = 291 therapeutic ERCPs, and n = 26 diagnostic ERCPs) found that the most common complication following ERCP in patients with PSC was pancreatitis (12 %), followed by cholangitis exacerbation (3 %), sepsis (3 %), duct perforation (2 %), post sphincterotomy bleeding (2 %) and liver abscess (1 %) [19]. A single-center retrospective cohort study comparing consecutive ERCP outcomes in patients with PSC (n = 30, total 85 ERCPs) and those with other biliary strictures (n = 45, total 70 ERCPs) over a 2-year period found no significant difference in the complication rates on a patient-based analysis (PSC 26.7 % [8/30]) versus non-PSC 13.3 % (6/45, p = 0.23) and on a per procedure base analysis (PSC 12.9 % [11/85]) versus non-PSC 8.6 % (6/70, P = .45). However, PSC patients with acute symptoms had a higher rate of complications than those whose procedures were done electively. There was a possible trend toward a higher incidence of cholangitis after therapeutic ERCP in PSC compared to non-PSC patients (7.8 % [5/64] versus 1.4 % [1/69], P = 0.11), despite a significantly higher rate of post-procedure antibiotic usage in the PSC cohort (P = .001) [4].
A retrospective study from Mayo clinic noted that the overall ERCP-related complications in patients with PSC (11 %; 18/168 patients) were not significantly different when compared to non-PSC patients (8 %;76/981; p = 0.2). The duration of hospitalization, complications such as perforation, pancreatitis, and bleeding were not different between PSC and non-PSC groups. However, the incidence of cholangitis was higher in PSC patients (4 %) compared to non-PSC patients (0.2 %), p < 0.0002 despite routine use of antibiotics. Compared to the non-PSC group (n = 981), the PSC group (n = 168) had a longer procedure duration (51 min ± 29 vs. 86 min ± 28, P = 0.02), a higher prevalence of portal hypertension (4 % vs. 31.5 %, p < 0.0001), underwent more biopsies (15 % vs. 39 %, p < 0.0001), had more brushings (8 % vs. 37 %, p < 0.001), underwent more balloon dilatations (15 % vs. 48 %, p < 0.0001) and had more intra-ductal ultrasounds (5 % vs. 11 %, p = 0.007) [31].
Predictors of ERCP Complications
A large multivariate analysis of 11,497 ERCP procedures done over a period of 12 years noted a total of 462 complications (4 %), of which 42 were severe (0.36 %) and 7 were fatal (0.06 %). Post-ERCP pancreatitis risk of 2.6 % and bleeding risk of 0.3 % were identified. Overall complications following ERCP were higher among individuals after a biliary sphincterotomy (odds ratio [OR] 1.32). Patients who had a history of chronic pancreatitis and those who received prophylactic pancreatic stenting had fewer complications (OR of 0.78 and 0.69 respectively). Bleeding risk was high after biliary sphincterotomy (OR 4.71]). Severe or fatal complications following ERCP were associated with severe (OR 2.38) and incapacitating (OR 7.65) systemic disease, obesity (OR 5.18), known or suspected bile duct stones (OR 4.08) and complex (grade-3) procedures (OR 2.86) [45].
Risk Factors for Post-ERCP Pancreatitis (PEP) in PSC
A retrospective study from Finland has noted an overall complication rate of 9 % (PEP 7 %, cholangitis 1.4 %, perforation 0.6 %, bleeding or death 0 %) in n = 389 consecutive PSC patients who underwent 441 total ERCP procedures with the guidewire cannulation technique. For patients with an intact papilla, the post-ERCP pancreatitis (PEP) rate was higher compared to those who had previous sphincterotomies (9.2 vs. 2.7 %; p = 0.01). Female sex (OR 2.6, p = 0.015), guide wire insertion into the pancreatic duct (OR 8.2, p < 0.01), and difficulties with cannulation were all associated with PEP. The incidence of PEP was 2.6 % when the pancreatic duct remained untouched compared to 20 % and 31.6 % incidence when the guide wire was inserted into the pancreatic duct twice or five times, respectively. The incidence of PEP was only 1.4 % if cannulation was performed without sphincterotomy. However the risk for PEP increased to 6.8 % with biliary sphincterotomy, 27 % with dual (pancreatic and biliary) sphincterotomies and up to 55.6 % with precut dual sphincterotomies [46].
Differential Diagnosis
Secondary Sclerosing Cholangitis
Secondary sclerosing cholangitis is also characterized by a similar multifocal biliary stricturing process due to identifiable causes (Table 22.1) that can mimic PSC in the both clinical and cholangiographic findings [25].
Secondary causes for sclerosing cholangitis |
|---|
Cholangiocarcinoma |
AIDS cholangiopathy |
IgG4 -associated cholangitis |
Ischemic cholangitis |
Portal hypertensive biliopathy |
Surgical biliary trauma |
Choledocholithiasis |
Eosinophilic cholangitis |
Recurrent pancreatitis |
Recurrent pyogenic cholangitis |
Hepatic inflammatory pseudotumor |
Histocytosis X |
Intra-arterial chemotherapy |
Mast cell cholangiopathy |
ABCB4 associated cholangiopathy |
Sclerosing cholangitis of critical illness |
Hypereosinophilic syndrome |
Sarcoidosis |
Graft-versus-host disease |
Amyloidosis |
Caroli’s disease |
Other types of ductal plate abnormalities |
Hodgkin’s disease |
Cholangitis glandularis proliferans |
Neoplastic/metastatic disease |
Hepatic allograft rejection |
Combined immunodeficiencies |
Angioimmunoblastic lymphadenopathy |
Congenital hepatic fibrosis |
Small Duct Primary Sclerosing Cholangitis
Population-based studies have noted that small duct PSC represents approximately 11–17 % of all patients with PSC [5, 9]. Small duct PSC patients have clinical, biochemical and histological features of PSC in the setting of a normal cholangiogram, although subtle changes can sometimes be seen in the small branches. The majority of patients with small duct PSC (>80 %) are noted to have associated IBD. Long-term follow-up studies have shown approximately 23 % of small duct PSC can progress to large duct PSC over time. Cholangiocarcinoma does not seem to occur in patients with small duct PSC, in the absence of progression to large duct PSC. Overall small duct PSC has a better long-term prognosis compared to large duct PSC [47].
PSC-AIH Overlap Syndrome
PSC-AIH (autoimmune hepatitis) overlap syndrome is most commonly diagnosed in young adults and children. The term “autoimmune sclerosing cholangitis” (ASC) has been proposed given the typical cholangiography finding of sclerosing cholangitis overlapping with the clinical, biochemical and histological features characteristic of autoimmune hepatitis [48].
This variant of PSC is diagnosed in 1.4–17 % of patients with PSC [49, 50]. Liver biopsy should be considered for the patients with disproportionately elevated aminotransferases (5- to 10-fold increase), increased level of serum auto-antibodies and/or hypogammaglobulinemia, with typical cholangiographic findings of PSC to diagnose or exclude overlap syndrome [6, 25]. Ursodeoxycholic acid has been used in combination with immunosuppressive drugs in the treatment of AIH-PSC overlap syndrome, and the long-term course has been considered favorable [50].
Immunoglobulin G4-Associated Cholangitis and PSC
Immunoglobulin G4-associated cholangitis (IAC) or IgG4-related cholangitis (IRSC) represents the biliary manifestation of a corticosteroid responsive systemic disease entity: IgG4-related disease (IgG4-RD). IgG4-RD could affect multiple organs, and is most often associated with increased serum IgG4 levels and characterized by IgG4 positive plasmacellular tissue infiltrates [51].
IAC affects mostly men (85 %) above middle age (mean age, 62 years), frequently presents with painless jaundice (77 %) and patients are less likely to have associated IBD. IAC has been noted to be associated with autoimmune pancreatitis (92 %), abundant IgG4-positive cells in bile duct biopsy specimens (88 %) and increased serum IgG4 levels (74 %) [52].
The current American Association for the Study of Liver Diseases (AASLD) practice guidelines recommend measurement of serum IgG4 in all PSC patients. If serum IgG4 is elevated, then evaluation for IAC for which a trial of steroid therapy is recommended [25]. Although IAC is usually responsive to corticosteroids, relapse is not uncommon after steroid withdrawal, particularly for patients with proximal bile duct strictures [6].
The interpretation of elevated serum IgG4 can be challenging considering that previous case-series have shown elevated IgG4 in 9–27 % of PSC patients without IAC or IRSC [53, 54]. A recent study from Europe noted that applying four times the upper limit of normal (4 × ULN) cut-off value for serum IgG4 (i.e., serum IgG4 > 5.6 g/L), was associated with the highest specificity and positive predictive value (100 %) for IAC, although sensitivity was low at 42 % (95 % CI 31–55) [51].
Cholangiocarcinoma
PSC should be considered a premalignant condition that warrants close surveillance given the risk of cholangiocarcinoma, which is 160-fold that of the general population [55–57].
A large retrospective study noted the median time from the diagnosis of PSC (n = 128) to cholangiocarcinoma (n = 26) was 26 months (range 0 months to 20.5 years). Forty-eight percent of the cases (n = 10) presented within 4 months of the diagnosis of PSC [36].
Based on the anatomic locations, cholangiocarcinoma can be divided into three subtypes: (1) intra-hepatic cholangiocarcinoma (iCCA), when located within the hepatic parenchyma; (2) perihilar cholangiocarcinoma (pCCA), when located proximal to the cystic duct; and (3) distal cholangiocarcinoma (dCCA), when located distal to the cystic duct [58]. The most common subtype is pCCA. In a large case series of patients with cholangiocarcinoma, 50 % had pCCA, 42 % had dCCA (42 %) and 8 % had iCCA [59].
The most commonly used staging system, the Bismuth-Corlette classification stratifies pCCA on the basis of bile duct involvement but it lacks crucial information such as vascular involvement or distant metastasis. Therefore this classification system was recently extended to also take into account vascular involvement (arterial/venous) and distal metastasis [60].
Cholangiocarcinoma often occurs at the site of dominant strictures in PSC patients [36, 61]. Dominant strictures are defined as stenosis ≤1.5 mm diameter in the common bile duct or ≤1 mm in a hepatic duct [25]. Therefore endoscopic brush cytology of a dominant stricture is advocated to diagnose cholangiocarcinoma (Fig. 22.6). However, the diagnosis of cholangiocarcinoma can be challenging because of its paucicellular nature, anatomic location and also because of the myriad of benign diseases that have clinical features suggestive of malignancy such as jaundice, abdominal pain, sudden change in liver biochemical tests and weight loss [58, 62]. Several studies have documented that positive cytology is highly predictive of presence of malignancy [63–67]. Unfortunately conventional brush cytology has a very low sensitivity (4 %–20 %) and low positive predictive value (≤60 %) despite its high specificity and high negative predictive values [19, 68]. The Mayo Clinic has reported that equivocal cytology results (atypical or suspicious) are much more common (approximately 40 %) than unequivocal positive cytology (<20 %) in diagnosing cholangiocarcinoma from their clinical experience [62]. Fluorescence in situ hybridization (FISH) and detecting aneuploidy using digital image analysis (DIA) are two advanced cytologic techniques that can increase the sensitivity of conventional cytology in diagnosing cholangiocarcinoma. FISH has been shown to increase the sensitivity up to 35–60 % while preserving specificity of cytology when assessing for polysomy (chromosomal gain). The sensitivity and specificity of DIA is intermediate compared with routine cytology and FISH but can have additive value when used along with FISH [62]. A small series, single center study has reported that in expert hands ERCP with probe-based confocal endomicroscopy had 100 % sensitivity (95 % CI 19.3–100 %) and 100 % negative predictive value (95 % CI 71.3.3–100 %) in excluding neoplasia. The specificity and positive predictive values were 61.1 % (95 % CI 35.8–82.6 %) and 22.2 % (95 % CI 3.5–59.9 %) respectively for this study [69]. Another recent, small single center prospective study has reported that cholangioscopy with narrow band imaging (NBI) did not improve the dysplasia detection rate compared to white light imaging despite increasing the biopsies (48 %) of suspicious lesions for patients with PSC [70]. Computed tomography (CT) or magnetic resonance imaging (MRI) may aid in the diagnosis of iCCA but liver biopsy is required for a definite diagnosis [58]. A diagnostic cut-off value of 130 U/ml for serum carbohydrate antigen (CA 19–9) tumor marker has a sensitivity and specificity of 79 % and 98 % respectively for diagnosing cholangiocarcinoma. However, CA 19–9 has a limited diagnostic use because it can also be increased in patients with bacterial cholangitis, significant intrahepatic cholestasis, and is virtually undetectable for those who are negative for Lewis antigen, which includes 7 % of the normal population.