Small Bowel Cancers
Kimberly Moore Dalal
Yuman Fong
Introduction
Small bowel cancers are relatively rare lesions, accounting for 2% of all gastrointestinal (GI) malignancies. Although most tumors are asymptomatic, some malignant masses only cause signs and symptoms after they have metastasized. Their rarity and insidious nature often lead to difficulty in diagnosis and discovery of disease at a late stage, resulting in a poor outcome.
Epidemiology and Etiology
Although 40% to 50% of all small bowel cancers are adenocarcinomas, 75% to 80% of these neoplasms are located in the duodenum and proximal jejunum. The U.S. incidence of small bowel adenocarcinomas is 0.46 per 100,000 and 0.33 per 100,000 for men and women, respectively. The second most common small bowel cancer is a carcinoid tumor, accounting for 35% of all small intestinal cancers; 90% are found in the ileum. Originating from the enterochromaffin cell, the annual U.S. incidence of carcinoids is 0.33 and 0.26 per 100,000 men and women, respectively. Lymphomas comprise the third major group of small intestinal tumors; these neoplasms may originate from the small intestine or may represent disseminated disease. In industrialized nations, 15% to 30% of small bowel cancers are classified as non-Hodgkin lymphomas (NHLs). Extranodal immunoproliferative B-cell lymphoma has been described in Arab and Jewish Middle Eastern populations (aka, “Mediterranean lymphoma”), North Africa, and South African blacks. Small bowel sarcomas account for 10% of small bowel cancers.
The risk of small intestinal cancers correlates positively with colorectal cancer, although the incidence is one-fiftieth of colorectal cancer in Western nations. This risk is attributed to high consumption of animal protein and fat.
Adenomatous polyps tend to occur in the periampullary region and proximal jejunum, close to the entrance of bile and pancreatic secretions into the small intestine. The adenoma-carcinoma sequence seen in colorectal cancer is also seen in small bowel cancers (1). The risk of adenocarcinoma increases in relation to increasing polyp size, villous features, and extent of epithelial dysplasia.
Several explanations may account for the low prevalence of carcinoma in the small intestine, an organ that comprises 75% of the length of the GI tract and 90% of the mucosal surface area. These include the presence of secretary immunoglobulin, intramural lymphoid tissue, rapid transit time of enteric contents that limits exposure of the mucosal surface to ingested carcinogens, high concentrations of pancreatic and biliary secretions, and lower concentrations of bacteria compared with the colon.
Clinical Presentation
The nonspecificity of symptoms and lack of physical signs explain the 6- to 8-month delay in diagnosis of small bowel cancers (2). For patients with malignant small bowel tumors, late or inaccurate diagnosis contributes to a 50% rate of metastasis at presentation (3,4).
Clinical features present at the time of diagnosis may include weight loss, malnutrition, anorexia, abdominal pain, nausea, vomiting, bleeding, or jaundice. More than 50% of patients present emergently with obstruction or bleeding. Perforation occurs in 10% of patients, particularly in those with lymphomas. Jaundice can occur with periampullary tumors or in patients with advanced liver metastases.
After a complete history, a palpable abdominal mass may be discovered in 25% of patients on physical examination. Rectal examination may reveal occult fecal blood.
Diagnostic Tests
Laboratory Tests
Laboratory tests should include a complete blood count, serum electrolytes, and liver function tests. Although no clear role for serum carcinoembryogenic antigen (CEA) has been demonstrated, the majority of small bowel adenocarcinomas are positive for CEA immunohistochemically (5). Patients with suspected small bowel carcinoids should undergo measurement of 24-hour urinary excretion of 5-hydroxyindoleacetic acid (5-HIAA), the end product of serotonin metabolism; this test has 75% sensitivity and 100% specificity (6). Most patients with carcinoid syndrome have values >100 mg/day (523 μm/day); lower levels (50–260 mg/day) may be seen in patients with metastatic carcinoid without the carcinoid syndrome. 5-HIAA levels correlate well with tumor mass (7). If 5-HIAA levels are nondiagnostic, measurements of urinary 5-hydroxytryptamine (5-HT, serotonin), serum 5-HT, serum chromogranin A, neuron-specific enolase, substance P, and neuropeptide K should be undertaken (see Chapter 48 for a complete review of carcinoid tumors).
Radiologic Studies
Abdominal plain films may reveal air-fluid levels or dilated bowel loops suggesting obstruction, or free air demonstrating perforation, but in general these films are not helpful.
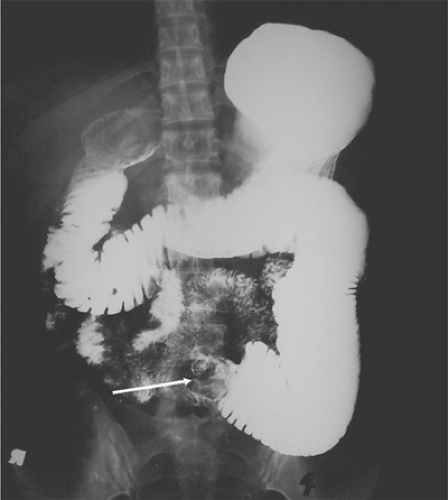
An upper gastrointestinal (UGI) series with small bowel follow-through (SBFT) with orally administered water-insoluble contrast is the traditional approach and has a sensitivity of 50% for small bowel tumors; they may show a mass lesion (Fig. 51.1), mucosal defect, or intussusception (Fig. 51.2).
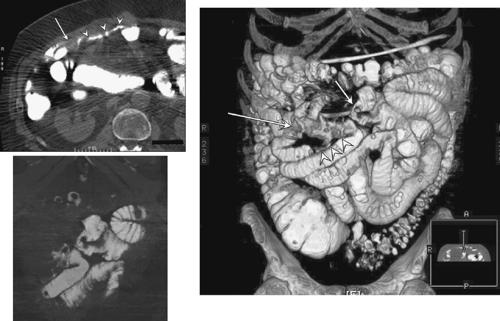
Enteroclysis is a double-contrast study in which a nasoenteric tube is advanced into the small bowel to a position above the suspected small bowel abnormality; this study is superior to UGI/SBFT for detecting small bowel tumors, with a sensitivity of 90% (8) except for flat infiltrating lesions, and is the diagnostic study of choice for small bowel tumors. Enteroclysis may be performed in conjunction with CT (9) and combines the benefits of cross-sectional imaging with barium contrast studies (Fig. 51.3). Only 50% to 60% of small bowel neoplasms are detected using UGI/SBFT or enteroclysis (4,10,11). Although enteroclysis may not allow visualization of a small carcinoid, mesenteric metastasis causing mass effect and angulation of bowel loops may suggest a possible diagnosis of carcinoid.
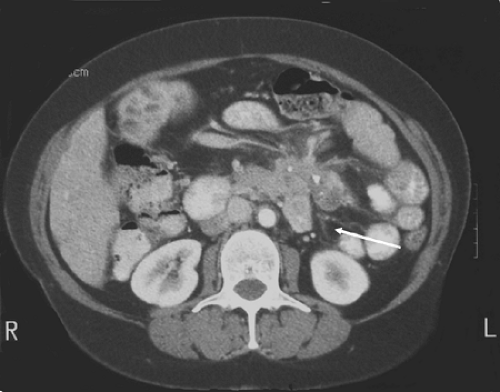
More commonly, patients will undergo abdominal and pelvic computed tomography (CT) for abdominal symptoms. CT has a sensitivity of 80% to 97% (4,10,11) and can evaluate proximal jejunal lesions often missed in contrast studies. CT may demonstrate a gastrointestinal stromal tumor (GIST) as a heterogeneous mass with focal areas of necrosis. In addition, CT can reveal the extraluminal extent of disease, as well as the lymphatic or intra-abdominal spread of disease. Magnetic resonance imaging is more costly than CT and in general is not more helpful in evaluating small bowel tumors. Finally, CT with intravenous and oral contrast is the procedure of choice for staging for carcinoid tumors and has an 87% sensitivity for identifying the primary tumor, mesenteric stranding and desmoplastic reaction, liver metastases, or mesenteric lymph node involvement (11) (Fig. 51.4).
UGI endoscopy and small bowel “push” enteroscopy are most helpful in delineating duodenal or proximal jejunal lesions, especially when radiographic evaluation has been unrevealing. One may obtain tissue diagnosis via endoscopic biopsy and provide therapy in selected instances (12). Push enteroscopy, which uses a pediatric colonoscope and allows visualization of the proximal 60 cm of jejunum, can establish the diagnosis in 50% of patients with obscure GI bleeding (13). In addition, extended small bowel enteroscopy using a 120-degree, forward-viewing, 2,560-mm balloon-tipped endoscope can allow visualization of 70% of the small bowel mucosa. Capsule endoscopy is a relatively new technique that allows visualization of the entire small bowel and can aid in evaluation of bleeding lesions (14). The risk of this procedure, however, is of complete obstruction; therefore, gastroenterologists request a UGI/SBFT to evaluate the presence of a near-obstructing intestinal lesion before administering the capsule. To further evaluate bleeding lesions, tagged red blood cell scans can detect bleeding at a rate of 0.1 mL/minute but cannot accurately identify bleeding sites. Angiography requires a bleeding rate of 0.5 mL/minute, can often localize the bleeding site, and may demonstrate tumor blush in carcinoid and leiomyosarcoma.
Radionuclide imaging using indium-111 octreotide localizes carcinoids as carcinoid tumor cells contain somatostatin receptors. Octreotide imaging has a >90% sensitivity for identifying carcinoid tumors in patients with carcinoid syndrome and is superior to metaiodobenzylguanidine (MIBG) scintigraphy (15). Iodine-131 or -121 MIBG scans can identify primary or metastatic carcinoids in 50% to 60% of patients (16). Rarely, a patient will benefit from selective venous sampling if other localization studies prove unsuccessful.
Laparoscopy or laparotomy is the most sensitive diagnostic modality in diagnosing a patient with a small bowel tumor. Laparoscopy with intraoperative endoscopy should be
considered in a patient with occult GI bleeding, unexplained weight loss, or vague abdominal pain and an unrevealing workup. Laparoscopy also allows for tissue samples in order to make a diagnosis.
considered in a patient with occult GI bleeding, unexplained weight loss, or vague abdominal pain and an unrevealing workup. Laparoscopy also allows for tissue samples in order to make a diagnosis.
Adenocarcinoma
Adenocarcinomas comprise the most common type of malignant small bowel tumors. Forty percent are located in the duodenum and decrease in frequency as one progresses distally along the small intestine. The incidence of small bowel adenocarcinomas in the United States is rising in African Americans (17,18). Risk factors include adenomatous polyps, familial adenomatous polyposis, hereditary nonpolyposis colorectal cancer, and Crohn disease (19). The most commonly used staging system is the tumor, node, metastasis (TNM) system of the American Joint Commission on Cancer (20) (Table 51.1). Treatment is dictated by location. Before proceeding with aggressive resection, one must exclude hepatic or peritoneal metastases or extensive locoregional invasion that may preclude complete excision. Lymph node involvement does not preclude an attempt at curative resection; examination of ≥15 lymph nodes improves the prognostic discrimination of nodal staging (21).
Ampulla and Duodenum
Ampullary carcinoma is an uncommon malignancy, accounting for 6% of periampullary tumors (22), with an incidence of 5.7 cases per 1 million people (23). Ampullary tumors have a more favorable prognosis (24,25,26,27,28) compared with pancreatic or bile duct tumors, with median survival rates ranging from 30 to 50 months (29,30) and 5-year survival rates of 30% to 50% (31,32) in resected patients. In 1963, Whipple mused that the improved prognosis of ampullary cancers was due to its fungating nature, better differentiation, and decreased tendency toward lymphovascular invasion (33).
The ampulla is comprised of the junction of the pancreatic and common bile ducts that forms a 3-mm common intramucosal channel. Anatomical variability of this area is found in half of the population. Invasive carcinoma obliterates the site of origin and the defining anatomical landmarks (Fig. 51.5). Adjacent to some carcinomas are precursor neoplasia or dysplasia of the duodenal mucosa or ductal system. Ampullary cancers cause an early jaundice that results in earlier detection of disease compared with pancreatic tumors.
Adenocarcinomas of the duodenal ampulla represent a variety of tumors that have features of ductal or intestinal origin. Ampullary tumors are biologically more similar to intestinal than pancreatic cancers. In one study, 70% of cases had intestinal rather than pancreaticobiliary morphology (34). Moreover, the frequent finding of ampullary tumors in patients with familial adenomatous polyposis suggests similar genetic alterations and mechanisms of carcinogenesis in colonic and ampullary neoplasms. In addition, adenocarcinoma of the duodenum may
be characterized by mutations of the APC/B-catenin pathway (35). Finally, K-ras mutations occur early in ampullary carcinomas with a pattern and incidence (37%) similar to colon cancer (36). DPC4 tumor-suppressor gene is inactivated in more than half of pancreatic adenocarcinomas; yet, complete loss of DPC4 was identified in only 34% of ampullary invasive carcinomas (37).
be characterized by mutations of the APC/B-catenin pathway (35). Finally, K-ras mutations occur early in ampullary carcinomas with a pattern and incidence (37%) similar to colon cancer (36). DPC4 tumor-suppressor gene is inactivated in more than half of pancreatic adenocarcinomas; yet, complete loss of DPC4 was identified in only 34% of ampullary invasive carcinomas (37).
Table 51.1 2002 Tumor, Node, Metastasis Staging of Small Intestine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree