CHAPTER 103 Short Bowel Syndrome
ETIOLOGY
The major causes of SBS in adults are Crohn’s disease for which multiple intestinal resections have been performed; mesenteric infarction from venous or arterial thrombosis, arterial embolism, or midgut volvulus; massive enterectomy performed to manage traumatic injuries or tumor resection, and radiation injury (Table 103-1). The causes of SBS in the pediatric population are congenital abnormalities (see Chapter 96), including gastroschisis, intestinal atresia, malrotation, aganglionosis, and necrotizing enterocolitis. More than 90% of infants now survive the extensive intestinal resections required for these conditions, and these patients need careful follow-up for their SBS as they mature to adulthood. Intestinal failure also can result from chronic intestinal pseudo-obstruction syndrome in both adults and children (see Chapter 120), as well as from unclassified sprue in adults (see Chapter 104) and congenital villus atrophy in children.
Table 103-1 Causes of Short Bowel Syndrome and Intestinal Failure
| In Adults |
| In Children |
* Functional short bowel syndrome also can occur in conditions associated with severe malabsorption, in which the bowel length often is intact.
INCIDENCE AND PREVALENCE
The incidence of SBS is difficult to assess in the United States, because of a lack of a national registry for affected persons and a lack of prospective studies in defined populations of patients who have undergone extensive intestinal resections. The incidence of severe SBS necessitating long-term parenteral nutrition is estimated to be 2 to 4 cases per 1 million persons per year, based on multinational European data.1 It is estimated that between 10,000 and 20,000 patients in the United States follow a home parenteral nutrition regimen for SBS. Approximately 50% to 70% of patients with SBS who initially require parenteral nutrition can be weaned from this therapy and therefore might not be reflected in the prevalence estimates.2,3 Such patients often still require aggressive nutritional monitoring. The incidence and prevalence of SBS associated with Crohn’s disease are decreasing now that infliximab and strictureplasty have become commonplace.
PATHOPHYSIOLOGY
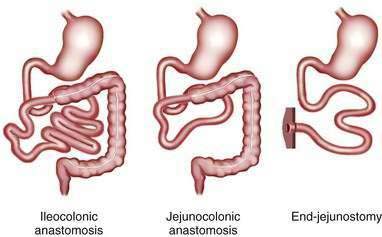
The major consequence of extensive intestinal resection is loss of absorptive surface area, which results in malabsorption of macronutrients, micronutrients, electrolytes, and water.4 The degree of malabsorption is determined by the length of the remnant intestine; the specific portions of small and large intestine resected, along with their site-specific transport processes and endocrine cells; and the adequacy of adaptive processes in the residual intestine over time. Three types of intestinal resections typically are encountered: limited ileal resection for Crohn’s disease, often with cecectomy or right hemicolectomy; extensive ileal resection with or without partial colectomy and with jejunocolonic anastomosis; and extensive small intestinal resection and total colectomy resulting in proximal jejunostomy (Fig. 103-1). Patients in the two latter groups commonly suffer from Crohn’s disease or had mesenteric infarction.
LOSS OF ABSORPTIVE SURFACE AREA
Nutrient Malabsorption
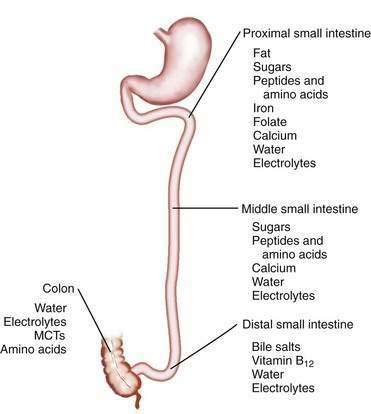
The length of the small intestine is estimated at 3 to 8 meters, and nutrient absorption is preserved until more than one half of the small intestine is resected.5–9 Most macronutrients (carbohydrate, fat, and nitrogen) are absorbed in the proximal 100 to 150 cm of intestine.10 Specific areas of absorption in the small intestine of nutrients, minerals, vitamins, electrolytes, and trace elements are discussed in Chapters 99 to 101 and are illustrated in Figure 103-2. Enterocytes lining the small intestine appear uniform from the duodenum to the ileocecal valve, but a distinct proximal-to-distal gradient exists in both morphology and function.11 Villi are taller and crypts are deeper in the jejunum than in the ileum, and the activity of microvillus enzymes and nutrient absorptive capacity per unit length of intestine are several-fold higher in the proximal than in the distal small intestine; loss of part of the jejunum initially compromises nutrient absorption more than does loss of an ileal segment of similar length, because of these morphologic and functional differences. The ileum, however, eventually is able to compensate for jejunal loss, whereas the jejunum is unable to compensate for ileal absorption of bile salts and vitamin B12.
Normal digestion and absorption depend on the gradual gastric emptying of partially digested nutrients, mixing of these nutrients with bile and pancreatic enzymes in the duodenum, and rapid digestion and absorption of the digestive products in the proximal small intestine. Patients with a proximal jejunostomy have rapid gastric emptying of liquids and rapid intestinal transit, which can compromise the gastric phase of digestion and result in inadequate mixing with biliary and pancreatic secretions, insufficient enzymatic digestion, and nutrient maldigestion. Rapid intestinal transit decreases nutrient-enterocyte contact time, and therefore, segmental absorption is decreased. Patients with a high jejunostomy are net secretors of salt and fluid, because jejunal fluid secretion is stimulated by oral intake and subsequent gastric emptying of nutrients; these patients excrete more fluid than they ingest, and accordingly, their fluid management may be challenging.12
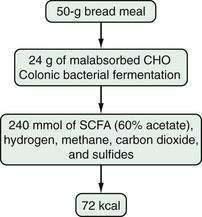
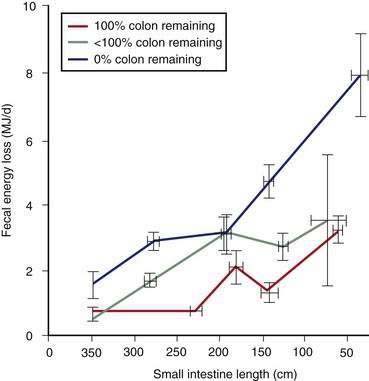
Most patients whose jejunum is shorter than 100 cm and who have no colon require long-term parenteral nutrition. Preservation of even some colon at surgery is highly beneficial for nutrient absorption. The ileocecal valve acts as a brake to slow intestinal transit, thereby increasing nutrient-enterocyte contact time and enhancing absorption. In addition, malabsorbed carbohydrates are fermented by bacterial enzymes in the colon to short-chain fatty acids (SCFAs), which are readily absorbed and used by colonocytes (Fig. 103-3). It has been estimated that this intracolonic digestive process can generate up to 1000 kcal (4.2 MJ) per day; in energy supply (Fig. 103-4), 1.0 MJ equals 238.8 kcal.13–15 Small intestine should be anastomosed to colon as soon as the patient is stable.
Water and Electrolyte Malabsorption
Loss of intestinal absorptive surface area can result in significant stomal or fecal losses of electrolytes, water, minerals, and trace elements (Table 103-2). The proximal small bowel receives approximately 7 to 9 L daily of water and electrolytes from food and secretions each day, of which 6 to 8 L are reabsorbed (see Chapter 99). On unrestricted diets, patients with a proximal jejunostomy cannot reabsorb such large volumes, a consequence of which is that voluminous diarrhea develops, often complicated by hypovolemia, hyponatremia, and hypokalemia. For example, in one study,12 the diarrheal volume in six jejunostomy patients with a mean jejunal length of 50 cm ranged from 3.2 to 8.3 L/day when they were allowed free access to food and water. All six patients were in negative sodium and water balance, four of the six were in negative potassium balance, and all six required parenteral nutrition with electrolyte replacement and restriction of oral intake of food and water to avoid unacceptable stomal losses. In the same study,12 seven of nine other jejunostomy patients who had a mean jejunal length of 120 cm were able to maintain positive water and sodium balance under the same conditions; absorption of water, sodium, and potassium in these 15 jejunostomy patients was correlated with jejunal length. At least 100 cm of intact jejunum is required to maintain positive water and electrolyte balance, similar to the length of jejunum required for nutrient absorption.
Table 103-2 Daily Stomal or Fecal Losses of Electrolytes, Minerals, and Trace Elements in Severe Short Bowel Syndrome*
| COMPONENT | AMOUNT LOST |
|---|---|
| Sodium | 90-100 mEq/L |
| Potassium | 10-20 mEq/L |
| Calcium | 772 (591-950) mg/day |
| Magnesium | 328 (263-419) mg/day |
| Iron | 11 (7-15) mg/day |
| Zinc | 12 (10-14) mg/day |
| Copper | 1.5 (0.5-2.3) mg/day |
* For sodium and potassium, the average concentration per liter of stomal effluent is given. The values for minerals and trace elements are mean 24-hour losses, with the range in parentheses. See text for details.
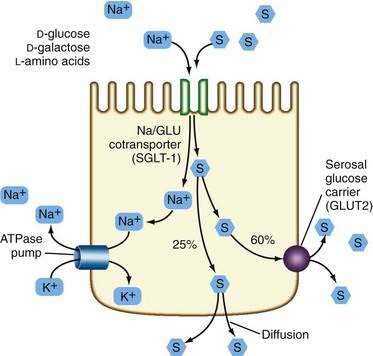
In general, patients with a proximal jejunostomy lose 90 to 100 mEq sodium and 10 to 20 mEq potassium per liter of stomal effluent (see Table 103-2).16 Some of these patients require long-term parenteral electrolyte and water supplements, often administered overnight, but others can maintain a positive balance by sipping a glucose-saline oral rehydration solution (ORS) throughout the day. The tight junctions of the jejunum are relatively leaky compared with the tight junctions of the ileum and colon, and therefore, a high NaCl concentration (greater than 90 mmol/L) is required in the glucose-saline solution to achieve net sodium and water absorption.17,18 Actively absorbed solutes (e.g., glucose, glucose polymers, galactose, oligopeptides, or l-amino acids) promote intestinal ion transport, although solutes also may be absorbed passively by means of solvent drag once active electrogenic Na+ absorption occurs.
Water transport into the enterocyte is directly proportional to Na+ transport. Na+ also is absorbed by means of an active electrogenic mechanism coupled with Cl− and H+ exchange and solvent drag. Absorptive and secretory processes occur simultaneously. Active Na+ secretion occurs against a concentration gradient from the enterocyte by means of the sodium pump, activated by Na+K+-ATPase (adenosine triphosphatase) in the basolateral membrane (Fig. 103-5). A mixture of 90 to 120 mmol/L NaCl and 50 mmol/L glucose is recommended, although such a solution might not be palatable. This mixture takes advantage of the coupled active transport of sodium with glucose and amino acids in the jejunum (see Chapter 99). Electrolyte and water absorption continue in the colon, and in normal humans only 100 to 150 mL of water is lost in the stool each day. The colon has a large reserve absorptive capacity for electrolytes and water, estimated to be 3 to 4 L of isotonic salt solution per day. Preservation of even part of the colon can reduce fecal electrolyte and water losses significantly in patients with SBS. A comparison of two groups of patients with similar jejunal length and jejunum either ending in a jejunostomy or anastomosis to colon showed that patients in the latter group were less likely to require oral or intravenous supplements.19
LOSS OF SITE-SPECIFIC TRANSPORT PROCESSES
Nutrient absorption potentially takes place at any level of the small intestine, albeit at different rates, owing to the proximal-to-distal gradient in functional activity of microvillus enzymes and transporters. The absorption of some compounds is restricted to certain areas of the small intestine (see Fig. 103-2); thus, calcium, magnesium, phosphorus, iron, and the water- and fat-soluble vitamins are absorbed predominantly in the duodenum and proximal jejunum (see Chapters 99 and 100).
Most patients with SBS have an intact duodenum and a variable length of jejunum, so the development of iron, phosphorus, or water-soluble vitamin deficiency, even in patients with a proximal jejunostomy, is relatively uncommon. Calcium absorption was found to be highly variable in a large study of patients with small intestinal resections.20 The net absorption of calcium (intake minus fecal loss) ranged from +573 to −268 mg/day, with a median of +65 mg/day; 64% of the patients, however, were in a negative calcium balance (intake minus fecal and urinary loss). In a study of 25 patients with a mean jejunal length of 128 cm, large-volume diarrhea (2 to 6 L/day), and steatorrhea,21 hypocalcemia and hypomagnesemia developed in 13 and 18 patients, respectively, during a trial of enteral hyperalimentation—despite supplementation with calcium, magnesium, and vitamin D. Malabsorption of calcium and magnesium is a consequence of fat malabsorption, because these minerals are precipitated intraluminally by unabsorbed long-chain fatty acids. Calcium and magnesium absorption improve on a low-fat diet in patients with small intestinal resections.22
The active absorption of vitamin B12 and bile acids is restricted to the ileum. The B12-intrinsic factor complexes and bile acids are taken up by specific transport proteins in ileal enterocytes (see Chapters 64 and 100). Most patients with SBS have lost part or all of the ileum, as a result of which vitamin B12 and bile acid malabsorption develops. The degree of malabsorption depends on the length of resected ileum. Vitamin B12 malabsorption usually is demonstrable when more than 60 cm of ileum has been resected.4 Resection of less than 100 cm of ileum causes moderate bile acid malabsorption and increased bile acid loss to the colon or in stomal effluents.23 The increased loss of bile acids to the colon induces electrolyte and water secretion and can exacerbate diarrhea. More-extensive ileal resections (greater than 100 cm) cause severe bile acid malabsorption, which, if bile acid loss exceeds hepatic synthesis, can result in a reduced bile acid pool size, with insufficient micellar solubilization of lipolytic products and resultant steatorrhea. Following extensive ileal resection, fat malabsorption develops, as can fat-soluble vitamin deficiency; essential fatty acid (linoleic acid) deficiency is rare. Loss of unabsorbed long-chain fatty acids to the colon can exacerbate diarrhea if the fatty acids are hydroxylated by colonic bacteria, because hydroxylated fatty acids stimulate colonic electrolyte and water secretion.24
LOSS OF SITE-SPECIFIC ENDOCRINE CELLS AND GASTROINTESTINAL HORMONES
The synthesis of gastrointestinal hormones in the intestinal mucosa is distributed in a site-specific manner along the gastrointestinal tract (see Chapter 1). Gastrin, cholecystokinin (CCK), secretin, gastric inhibitory polypeptide, and motilin are produced by endocrine cells in the proximal gastrointestinal tract and regulate secretory processes and motility. The area within which these hormones are synthesized usually is intact in patients with SBS, and hormonal profiles are normal. In approximately 50% of patients with extensive intestinal resections, however, hypergastrinemia and increased gastric acid secretion temporarily develop in the early postoperative phase.25,26 The cause of this postoperative hypergastrinemia is not known but could be loss of inhibitory signals, because it resolves spontaneously.
Glucagon-like peptides 1 and 2 (GLP-1 and GLP-2), neurotensin, and peptide YY (PYY) are produced in the ileum and proximal colon, and these intestinal segments are often lost in SBS patients.27 GLP-1 and GLP-2 and PYY are released by intraluminal fat and carbohydrates, cause a delay in gastric emptying, and slow intestinal transit (the ileal brake).28,29 Jejunostomy patients demonstrate impaired release of these hormones in response to a meal, rapid gastric emptying, and rapid intestinal transit of liquids.30,31 Patients with SBS and preserved colon have increased GLP-1 and GLP-2 concentrations and demonstrate normal gastric emptying.32 GLP-1 and GLP-2 and PYY also have been shown to inhibit gastric acid secretion and to promote intestinal growth in animal models.
LOSS OF THE ILEOCECAL VALVE
The primary functions of the ileocecal valve are to separate ileal and colonic contents, thereby minimizing bacterial colonization of the small intestine, and to regulate emptying of ileal contents into the colon. The ileocecal valve is removed in most ileal resections, as a consequence of which intestinal transit time decreases, and bacterial overgrowth is risked if the ileum is anastomosed to the colon. Bacterial overgrowth can worsen nutrient and cobalamin malabsorption (see Chapters 100 and 102), because bacteria compete with enterocytes for nutrient assimilation. Rapid intestinal transit in these patients, however, can counteract the risk of bacterial colonization. Studies are lacking to document the role of bacterial overgrowth in malabsorption in patients with SBS.
INTESTINAL ADAPTATION TO RESECTION
Adaptive changes in the remaining intestine after intestinal resection have been studied extensively in animal models and to a limited extent in humans33,34; adaptive changes are more pronounced in the ileum than in the jejunum. After jejunectomy and duodenoileal anastomosis, the ileum attains the morphologic characteristics of the jejunum, with taller villi and deeper crypts35; with time, an increase in ileal diameter and length also occurs. A prospective study of seven patients with jejunoileal bypass operation (20 cm of jejunum anastomosed to 25 cm of ileum) showed an increase in the length and diameter of the jejunum (80% and 40%, respectively) and ileum (128% and 50%, respectively) after 18 months of observation.36 An increase in absorptive capacity was demonstrated in another study of 41 patients with SBS (mean jejunal length, 119 cm) in whom the mean stool volume decreased from 2.5 to 0.9 L per day over a period of 3 months with continuous oral intake37; patients gained weight, and nitrogen balance increased from +3.2 g in the first month to +7.8 g in the second month postoperatively. The same study also demonstrated a gradual increase in intestinal transit time, which was most pronounced for ileal transit. The result of all of these changes is an increase in intestinal absorptive surface area, with an increase in microvillus enzyme activity and absorptive capacity per unit length of intestine.38 An improvement in mineral absorption with time also has been observed in a series of 30 patients with SBS (mean jejunal length, 81 cm) in whom fractional calcium absorption was correlated with time after surgery.39
In humans, these adaptive changes can take 1 to 2 years to develop fully. The younger the patient, the more profound the adaptive response. Adaptive changes depend on the presence of food and biliary and pancreatic secretions in the intestinal lumen40; adaptive hyperplasia of the ileum failed to develop in jejunectomized animals fed only by parenteral alimentation.41 To induce these adaptive processes, patients with SBS are encouraged to start oral intake as early as possible in the postoperative phase. Patients with SBS whose colon is in continuity demonstrate qualitative and quantitative changes in colonic flora that result in an increased capacity to metabolize carbohydrate and in an increased fecal bacterial mass.42
Adaptive hyperplasia is the result of an increase in crypt cell production rate, presumably mediated by growth factors released by the presence of food and secretions in the intestinal lumen. Vascular endothelial growth factor (VEGF), CCK, gastrin, insulin, neurotensin, GLP-2, and l-glutamine have been shown to stimulate intestinal growth in experimental animals;43–45 studies in humans have not indicated any value of supplemental glutamine to enhance intestinal adaptation.46,47 These extracellular growth factors stimulate polyamine synthesis in crypt cells, which in turn induces increased DNA synthesis and mitotic activity.48 Inhibition of polyamine synthesis in jejunectomized animals prevents adaptive changes in the ileum.49 Elucidation of the mediators regulating enterocyte proliferation eventually can lead to development of pharmacologic interventions that can accelerate intestinal adaptation in patients with SBS. The presence of comorbid conditions and the health of the residual bowel and its blood flow are important factors in the prognosis for patients who have undergone massive enterectomy.
MEDICAL MANAGEMENT
The initial management of the patient with SBS involves primarily supportive care designed to enhance the potential for survival. This care includes achievement of hemodynamic stability and appropriate fluid and electrolyte management. In the immediate postoperative phase, most patients with extensive intestinal resections are kept fasting and are supported with total parenteral nutrition (TPN). Weight and volume status are carefully monitored and stomal, fecal, and urinary losses of water, sodium, and potassium are measured to ensure optimal electrolyte and water balance. Histamine H2 receptor blockers or proton pump inhibitors are given intravenously to suppress hypergastrinemia-induced gastric acid hypersecretion and to limit volume losses.50,51 Patients with jejunostomies have stomal effluents up to several liters per day in this early phase, with obligatory losses of sodium, potassium, and possibly magnesium. Enteral tube feeding, followed by oral feeding, is begun in the late postoperative phase once the patient is hemodynamically stable, adequate intestinal blood flow has been restored, and postoperative ileus has resolved. Patients with extensive resections are kept fasting up to 5 to 10 days to allow a second-look operation at 24 to 48 hours, for the healing of enteric anastomoses, and to assess basal losses of water and electrolytes.
LIMITED ILEAL RESECTION
Patients with a limited ileal resection (less than 100 cm), with or without right hemicolectomy, may resume intake of solid food in the late postoperative phase. The response to solid food is determined mainly by the length of ileum removed and whether or not the right colon was resected; patients can develop diarrhea or steatorrhea with consumption of a regular diet. Secretory diarrhea without steatorrhea is the typical finding in limited ileal resections. Treatment with a bile acid-binding resin, such as cholestyramine (2-4 g with meals) or colestipol (1-2 g with meals) often ameliorates diarrhea if bile acid malabsorption is the main cause. Colestipol often is tolerated better than is cholestyramine. The diarrhea of some patients with limited ileal resection and right hemicolectomy does not respond to cholestyramine or colestipol despite documented bile acid malabsorption and presumably is due to loss of intestinal absorptive capacity for sodium chloride.52
Patients with documented fat malabsorption on a regular diet might have less severe steatorrhea while on a low-fat (40 g), high-carbohydrate diet; however, oral energy intake also will be reduced, because fat is calorically dense (9 kcal/g). Patients maintained on such a diet experience a decrease in diarrhea and steatorrhea and improve their net absorption of calcium, magnesium, and zinc.4 If necessary, medium-chain triglycerides (MCTs), which do not require micellar solubilization, can be added as a source of fat calories. The possibility of vitamin B12 malabsorption should be assessed with a Schilling test, and if the malabsorption is documented, parenteral B12, usually in a dose of 1 mg intramuscularly monthly, is required for life.
Malabsorption of fat-soluble vitamins, calcium, and magnesium is a risk in patients with fat malabsorption. Fourteen of 27 patients with ileal resections of 50 to 150 cm and an intact colon were in negative calcium balance when studied on a fixed daily calcium intake of 800 mg supplemented with 400 to 800 IU of vitamin D daily.21 Supplementation with vitamins, calcium, and possibly magnesium should be initiated before overt signs of vitamin deficiency or hypocalcemia and hypomagnesemia develop. Magnesium supplementation by mouth may be unrewarding, because magnesium is a cathartic. Although magnesium gluconate is water soluble and therefore may be the most readily absorbed magnesium salt, some patients still require periodic parenteral replacement. Magnesium deficiency can occur despite a normal serum concentration, because most Mg2+ is present in the intracellular space. Therefore, measurement of 24-hour urine Mg2+ concentration is prudent in patients who have suspected magnesium deficiency but normal serum Mg2+ concentration. Magnesium deficiency can result in calcium deficiency, because the release of parathyroid hormone is impaired in the presence of hypomagnesemia.53
Most patients with SBS already are in a negative calcium balance54 and therefore an oral supplement of calcium at a daily dose of 800 to 1500 mg is recommended. The tests to assess vitamin and mineral balance and recommended dosages in patients with malabsorption are discussed in Chapters 99 and 100. Absorption of water-soluble vitamins, carbohydrates, and proteins is, in general, not compromised in patients with limited ileal resections.
EXTENSIVE SMALL INTESTINAL RESECTION AND PARTIAL COLECTOMY
Management of Fluid and Electrolytes
Massive enterectomy is associated with gastric hypersecretion for approximately the first six months postoperatively. These patients benefit from the use of intravenous H2 antagonists or oral or intravenous proton pump inhibitors; absorption of orally ingested medications may be impaired, and more than the usual doses of these agents may be required (Table 103-3). Rapid intestinal transit contributes to malabsorption and diarrhea, and use of antidiarrheal drugs is common (see Table 103-3). These medications should be taken one hour before meals, and their effect on volume of diarrhea should be evaluated before long-term treatment is recommended.
Table 103-3 Therapeutic Agents Used to Decrease Intestinal Transit and Diarrheal Volume
| AGENT | DOSAGE |
|---|---|
| Loperamide* | 4-6 mg four times daily |
| Diphenoxylate-atropine* | 2.5-5 mg four times daily |
| Codeine phosphate* | 15-30 mg two to four times daily |
| Tincture of opium | 0.6 mL (2.5 mg) two to four times daily |
| Ranitidine† | 300 mg twice daily |
| Omeprazole‡ | 40 mg twice daily |
| Octreotide | 50-100 µg SC twice daily |
| Clonidine | 0.3 mg transcutaneous patch once weekly |
SC, subcutaneously.
* The antidiarrheal agents loperamide, diphenoxylate-atropine, and codeine are given 1 hour before meals and at bedtime. Dosages may be increased over those recommended, because of incomplete absorption in patients with short bowel syndrome.
† Cimetidine, famotidine, and nizatidine are alternatives.
‡ Esomeprazole, lansoprazole, rabeprazole, and pantoprazole are alternatives.
Use of antimotility agents is important to control fluid losses; such agents include loperamide hydrochloride (16 to 24 mg/day) and diphenoxylate (10 to 20 mg/day), codeine (30 to 240 mg/day), tincture of opium, and the somatostatin analog octreotide (50 to 100 µg two or three times a day). Most studies have shown that these agents reduce stomal output by up to 50%, but a positive water and electrolyte balance rarely is achieved. Octreotide usually is not necessary except for some patients with a proximal jejunostomy. Octreotide can slow intestinal transit and increase sodium and water absorption,55–58 but it also decreases splanchnic protein synthesis, thereby inhibiting postresectional intestinal adaptation57; the risk of cholelithiasis also is increased with octreotide.58 The α2-adrenergic agonist clonidine also may be useful to decrease diarrhea by its effects on chloride absorption. Transdermal administration avoids the potential for medication malabsorption.59 Glucose polymer-based ORSs should be provided to patients to improve hydration and thereby reduce TPN requirements.
Glucose and sodium are absorbed by the same active transport mechanism and stimulate absorption of each other. In addition, glucose promotes sodium and water absorption by means of solvent drag (see Fig. 103-5).60 Therefore, because the jejunum is permeable to both sodium and chloride, passively absorbed solutions that have a high sodium chloride concentration are absorbed to a significant degree; sodium is not as readily absorbed from isotonic or hypotonic solutions. A simple solution developed by the World Health Organization (WHO) can be formulated by dissolving 2.5 g of table salt, 1.5 g of KCl (requires a prescription), 2.5 g of sodium bicarbonate (NaHCO3), and 1.5 g of table sugar (sucrose) in 1 L of water. This solution provides a sodium concentration of approximately 90 mmol/L. Additional salt may be added to increase the osmolarity as tolerated, to 100 to 120 mmol/L or more, which can increase effectiveness.61 Sodium losses actually increase when solutions are consumed that contain less sodium than is in the small bowel effluent (90 mmol/L). The use of ORS is not as critical in patients in whom the colon is intact, provided that sufficient dietary sodium is present, because of the colon’s ability to absorb sodium and water. For patients who have had significant jejunal resections, the addition of glucose to the ORS is not critical, because glucose does not enhance ileal water absorption.62 In addition to sodium losses, significant quantities of bicarbonate and magnesium are lost in feces.