Chapter 6 Edward Zoltan, MD; Ross T. Cockrell, MD Sexually transmitted infections (STIs) remain a major public health challenge in the United States, affecting more than 20 million men and women annually, nearly half of whom are young people aged 15-24 years. In addition to the physical and psychologic consequences of STIs, these diseases exact a tremendous economic toll. Direct medical costs associated with STIs in the United States are estimated at $16 billion annually treating a total of 110 million STIs. The curable STIs include gonorrhea, chlamydia, mycoplasma, ureaplasmal infections, syphilis, trichomoniasis, chancroid, lymphogranuloma venereum (LGV), and donovanosis. STIs caused by yeast or protozoa are also curable. Viral STIs include human immunodeficiency virus (HIV), human papillomavirus (HPV), hepatitis B/C virus (HBV, HCV), cytomegalovirus (CMV), and herpes simplex virus (HSV); they are preventable but not curable. This introduction to STIs is organized and presented by pathogens. A focus on diagnosis and treatment with some epidemiology will be the blueprint for this chapter. Gonorrhea, Neisseria gonorrhoeae, is one of the most commonly reported infectious diseases in the United States, with 321,849 cases reported in 2011. Gonorrhea is thought to be substantially underdiagnosed and underreported; approximately twice as many new infections are estimated to occur each year as are reported. A direct Gram stain may be performed as soon as the specimen is collected. Urethral smears from men who have symptomatic gonorrhea usually contain intracellular gram-negative diplococci in polymorphonuclear leukocytes. Endocervical smears from women and rectal specimens require careful interpretation because of colonization with other gram-negative coccobacillary organisms. In addition, the Centers for Disease Control and Prevention (CDC) is now recommending annual screening for sexually active women with risk factors (i.e., multiple sexual partners, communities with high disease rate). Pregnant women at risk should also receive screening at first prenatal visit. Men who engage in sexual intercourse with other men should be screened more frequently, every 3 to 6 months if illicit drug use is found (especially methamphetamine). The isolation and identification of N. gonorrhoeae is still the currently accepted gold standard for the diagnosis of gonococcal infections. It is the recommended method for medicolegal investigations of sexual abuse. Specimens should be inoculated onto selective media, such as modified Thayer-Martin, Martin-Lewis, or New York City. Current testing for N. gonorrhoeae also includes nucleic acid molecular amplification tests (NAATs) that measure deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) rather than live organisms. Polymerase chain reaction (PCR) (Amplicor, Roche Molecular Diagnostics, Branchburg, N.J.) has been used with sensitivity well above 90% for the detection of N. gonorrhoeae in cervical specimens. It is highly accurate with male urine. Strand displacement amplification (SDA) (ProbeTec, Becton Dickinson, Sparks, Md.) is approved for detection of gonorrhea in cervical, male urethral, and female and male urine samples and has achieved widespread use in clinical laboratories throughout the United States and Europe. The BD ProbeTec ET system demonstrated a sensitivity of 97.9% for the detection of N. gonorrhoeae in urine from 680 male patients. Because NAATs measure DNA or RNA rather than live organisms, caution should be used in using DNA amplification tests for test-of-cure assays. Residual nucleic acid from cells rendered noninfective by antibiotics may give a positive amplified test for up to 3 weeks after therapy, although the patient may actually be cured of viable organisms. Ceftriaxone 250 mg intramuscularly (IM) in a single dose (99% effective) is the primary choice. If unavailable, Cefixime 400 mg orally in a single dose, or azithromycin 2 g oral in pregnant women who cannot tolerate cephalosporins plus, dual therapy to treat chlamydial infection is recommended because treating is more cost effective than testing for associated chlamydial infection: azithromycin 1 g orally in a single dose or doxycycline 100 mg orally twice a day for 7 days. Recently two new regimens have been released by the CDC: gentamicin with oral azithromycin (100% effective) and oral azithromycin with oral gemifloxacin (99.5% effective). Given increasing antimicrobial resistance, it is important to consistently consult current literature for updates in treatment. Apps are available from the CDC (www.cdc.gov/mobile/mobileapp.html). The CDC no longer recommends quinolones for gonorrhea infections because of new data showing increased resistance. In addition, use of quinolones is inadvisable for treating infections acquired in California and in other areas with increased prevalence of quinolone resistance. Patients who have uncomplicated gonorrhea and who are treated with any of the recommended regimens need not return for a test to confirm that they are cured. Patients who have symptoms that persist after treatment should be evaluated by culture for N. gonorrhoeae, and any gonococci isolated should be tested for antimicrobial susceptibility. Infections identified after treatment with one of the recommended regimens usually result from reinfection rather than treatment failure, indicating a need for improved patient education and referral of sex partners. Persistent urethritis, cervicitis, or proctitis may indicate infection by another organism, including Chlamydia trachomatis. All sexual partners who had contact within 60 days of diagnosis should be evaluated, tested, and treated for both N. gonorrhoeae and C. trachomatis. Sexual activity should be avoided until treatment is completed and symptoms have resolved in all partners. Complications of N. gonorrhoeae may be local or systemic. Locally, male GC urethritis may spread to the posterior urethra, seminal vesicles, and epididymis. This can lead to epididymitis, urethral stricture disease, prostatitis, and even sterility. In women, gonorrhea is a major cause of pelvic inflammatory disease (PID). PID results from the ascent of infection from the endocervix into the fallopian tubes. Symptoms include vaginal discharge, abdominal pain, dyspareunia, menorrhagia, fever, and cervical motion or adnexal tenderness. This may lead to scarring of the adnexal structures and fallopian tubes, resulting in chronic pelvic pain, ectopic pregnancy, and infertility. N. gonorrhoeae can also lead to systemic diseases. Gonococcal perihepatitis (Fitz-Hugh-Curtis syndrome) is manifested by sharp supraumbilical pain and right upper quadrant pain. It results from ascending infection from the pelvis into the paracolic gutters and subphrenic spaces in females. “Violin string” adhesions may be noted between the liver and anterior abdominal wall and diaphragm. Disseminated gonococcal infection can occur in up to 3% of mucosal cases and may include arthritis, tenosynovitis, dermatitis, meningitis, myopericarditis, and/or sepsis. Chlamydia, C. trachomatis, is the most frequently reported STI in the United States, with an estimated 4 million new cases each year. In 2011, 1.4 million chlamydia diagnoses were reported, up from 877,478 in 2003. National surveys indicate that chlamydia prevalence among sexually active females aged 14 to 19 is 6.8%, Even so, most chlamydia cases go undiagnosed. The increases in reported cases and rates likely reflect the continued expansion of screening efforts and increased use of more sensitive diagnostic tests, rather than an actual increase in new infections. The availability of urine tests for chlamydia may be contributing to the increased detection of the disease in men and consequently the rising rates of reported chlamydia in men in recent years. The CDC recommends annual chlamydia screening for sexually active women under age 25, all pregnant women, and older women with risk factors (i.e., new or multiple sex partners). Screening efforts are critical to preventing the serious health consequences of this infection, particularly infertility. Such efforts are linked to a 60% reduction in the incidence of PID. Screening of men who engage in sexual intercourse with other men should be conducted annually, with more frequent screening (every 3 to 6 months) for men involved with multiple partners. If illicit drug use is involved (especially methamphetamine), involved parties are at an even higher risk, and they should receive testing at shorter intervals. C. trachomatis is an intracellular bacterium with multiple serotypes. Types L1-3 cause LGV. Types D, E, F, G, H, I, J, and K cause urethritis and cervicitis. It is transmitted during vaginal, oral, or anal sexual contact with an infected partner. C. trachomatis accounts for 30% to 50% of cases of nongonococcal urethritis (NGU). Chlamydial infection in men is generally asymptomatic, but symptoms may include urethritis, epididymitis, and proctitis. Urethritis presents 1 to 3 weeks after infection with a mild to moderate clear urethral discharge and dysuria. Chlamydial urethritis may present as persistent dysuria and/or discharge following a course of treatment for gonorrhea because 15% to 35% of patients with known gonococcal infections are also infected with chlamydia. Chlamydial epididymitis tends to run a more protracted course than epididymitis because of other organisms; it is also often less severe. Chlamydial proctitis may present with rectal pain and bleeding, but it is often asymptomatic. Chlamydial infection may disseminate systemically in 1% to 3% of patients. Classically know as Reiter syndrome, it presents with the classic triad of reactive arthritis, urethritis, and conjunctivitis. The Fitz-Hugh-Curtis syndrome may also be seen in about 20% of women with PID secondary to chlamydial infection. C. trachomatis was the first organism for which there was a commercially available PCR assay. Now there are many published studies using several different types of NAATs and new technologies that are commercially available for detecting chlamydia in urine and urethral, cervical, or vaginal secretions. Treatment should be initiated as soon as possible after diagnosis. Single-dose regimens have the advantage of improved compliance. • Azithromycin 1 g orally in a single dose • Or doxycycline 100 mg orally twice a day for 7 days • Erythromycin base 500 mg orally four times a day for 7 days • Or erythromycin ethylsuccinate 800 mg orally four times a day for 7 days • Or ofloxacin 300 mg twice a day for 7 days • Or levofloxacin 500 mg once daily for 7 days Patients who have persistent or recurrent urethritis should be retreated with the initial regimen if they did not comply with the treatment regimen or if they were reexposed to an untreated sex partner. Otherwise, a culture of an intraurethral swab specimen and a first-void urine specimen for Trichomonas vaginalis should be performed. Some cases of recurrent urethritis following doxycycline treatment may be caused by tetracycline-resistant Ureaplasma urealyticum. If the patient was compliant with the initial regimen and reexposure can be excluded, the following regimen is recommended: Metronidazole 2 g orally in a single dose PLUS erythromycin base 500 mg orally four times a day for 7 days or erythromycin ethylsuccinate 800 mg orally four times a day for 7 days Patients who have NGU and also are infected with HIV should receive the same treatment regimen as those who are HIV negative. The etiologies of most cases of nonchlamydial nongonococcal urethritis are unknown. U. urealyticum and Mycoplasma genitalium have been implicated. Specific diagnostic tests for these organisms are not indicated because the detection of these organisms is often difficult and would not alter therapy. T. vaginalis and HSV sometimes cause urethritis. Diagnostic and treatment procedures for these organisms are reserved for situations in which these infections are suspected (e.g., contact with trichomoniasis and genital lesions suggestive of genital herpes) or when unresponsive to therapy. HPV is the most common STD with an estimated total of 20 million infected individuals in the United States. HPV vaccination has decreased prevalence by 56% in females aged 14 to 19. HPV, a small, nonenveloped virus containing double-stranded DNA, infects basal epithelial cells and multiplies in the cell nucleus, causing cell death and perinuclear cavitation or koilocytosis, a histologic feature specific to HPV. More than 40 types of HPV can infect the genital tract. Most HPV infections are asymptomatic, unrecognized, or subclinical. Visible genital warts usually are caused by HPV type 6 or 11 (90%). Other HPV types in the anogenital region (e.g., types 16, 18, 31, 33, and 35) have been strongly associated with cervical neoplasia. Biopsy is recommended under certain clinical conditions: 2. Lesions do not respond to standard therapy. 3. Disease worsens during therapy. 4. Patient is immunocompromised. 5. Warts are pigmented, indurated, fixed, and ulcerated (suggestive of Buschke-Löwenstein tumor). No data support the use of type-specific HPV nucleic acid tests in the routine diagnosis or management of visible genital warts. In addition to the external genitalia (i.e., the penis, vulva, scrotum, perineum, perianal skin), genital warts can occur on the uterine cervix and in the vagina, urethra, anus, and mouth. When urethral lesions occur, 80% are located within the distal 3 cm of urethra. Patients present with dysuria, bloody urethral discharge, and changes in urinary stream. Bladder condyloma are rare. If meatal condyloma are identified, urethroscopy should be performed to look for other urethral lesions. To prevent iatrogenic seeding of the prostatic urethra and bladder, a tourniquet should be placed at the penopubic junction, or urethroscopy should stop at the external sphincter. Intraanal warts are seen predominantly in patients who have had anal-receptive intercourse; these warts are distinct from perianal warts, which can occur in men and women who do not have a history of anal sex. HPV types 6 and 11 have been associated with conjunctival, nasal, oral, and laryngeal warts. HPV types 6 and 11 rarely are associated with invasive squamous cell carcinoma of the external genitalia. Depending on the size and anatomic location, genital warts can be painful, friable, and pruritic, although they are commonly asymptomatic. HPV types 16, 18, 31, 33, and 35 are found occasionally in visible genital warts and have been associated with external genital (i.e., vulvar, penile, anal) squamous intraepithelial neoplasia (i.e., squamous cell carcinoma in situ, bowenoid papulosis, erythroplasia of Queyrat, Bowen disease of the genitalia). These HPV types also have been associated with vaginal, anal, and cervical intraepithelial dysplasia and squamous cell carcinoma. Patients who have visible genital warts can be infected simultaneously with multiple HPV types (Figure 6-1). The introduction of two vaccines has resulted in a shift in focus from treatment of visible lesions to prevention: Cervarix, a bivalent HPV vaccine against Types 16 and 18 (approximately 70% of cervical cancers) and Gardasil a quadrivalent vaccine 16, 18, 6, and 11 (90% of genital warts). Both are administered in three-dose series and are routinely recommended for 11- and 12-year-old girls and boys (Gardasil is the only one FDA approved for boys) and can be started at 9 years of age. Gardasil is also recommended for females 13 to 26 and males 13 to 21 and may be given to 22- to 26-year-old males. Vaccination is also routinely recommended for gay and bisexual men and patients with compromised immune systems (including HIV) aged 22 to 26. Efficacy of the bivalent vaccine in women in preventing cervical cancer related to HPV 16 and 18 is nearly 93% where as the quadrivalent and demonstrated 100% efficacy in preventing cervical, vulvar, and vaginal precancers. However, vaccinations do not replace other prevention strategies. Otherwise, the primary goal of treating visible genital warts is the removal of symptomatic warts. In most patients, treatment can induce wart-free periods. If left untreated, visible genital warts may resolve spontaneously, remain unchanged, or increase in size or number. Existing data indicate that currently available therapies for genital warts may reduce but probably do not eliminate infectivity. Most patients have fewer than 10 genital warts, with a total wart area of 0.5 to 1.0 cm2. These warts respond to most treatment modalities. Factors that may influence the selection of treatment include wart size, wart number, anatomic site of wart, wart morphology, patient preference, cost of treatment, convenience, adverse effects, and provider experience. Many patients require a course of therapy rather than a single treatment. In general, warts located on moist surfaces and/or in intertriginous areas respond better to topical treatment than do warts on drier surfaces. Podofilox 0.5% solution or gel: Patients should apply podofilox solution with a cotton swab or podofilox gel with a finger to visible genital warts twice a day for 3 days, followed by 4 days of no therapy. This cycle may be repeated, as necessary, for up to four cycles. The total wart area treated should not exceed 10 cm2, and the total volume of podofilox should be limited to 0.5 mL per day. The safety of podofilox during pregnancy has not been established. Imiquimod 5% cream: Patients should apply imiquimod cream once daily at bedtime, three times a week for up to 16 weeks. The treatment area should be washed with soap and water 6 to 10 hours after the application. The safety of imiquimod during pregnancy has not been established. Sinecatechins 15% ointment: Patients should apply the ointment three times daily for up to 16 weeks. DO NOT wash off postapplication. The safety of sinecatechins during pregnancy or in HIV or HSV–co-infection has not been established. Cryotherapy with liquid nitrogen or cryoprobe: Repeat applications every 1 to 2 weeks. Podophyllin resin 10% to 25% in a compound tincture of benzoin: A small amount should be applied to each wart and allowed to air dry. The treatment can be repeated weekly if necessary. To avoid the possibility of complications associated with systemic absorption and toxicity, the application should be limited to less than 0.5 mL of podophyllin or an area of less than 10 cm2 of warts per session. The preparation should be thoroughly washed off 1 to 4 hours after application to reduce local irritation. The safety of podophyllin during pregnancy has not been established. Trichloroacetic acid (TCA) or bichloroacetic acid (BCA) 80% to 90%: A small amount should be applied only to warts and allowed to dry, at which time a white “frosting” develops. If an excess amount of acid is applied, the treated area should be powdered with talc, sodium bicarbonate (i.e., baking soda), or liquid-soap preparations to remove unreacted acid. This treatment can be repeated weekly if necessary. Intralesional interferon: Intralesional injection of interferon alpha 2b increases success of podofilox, but the recurrence rate is the same. Laser surgery: Carbon dioxide (CO2) laser vaporizes tissue with a shallow depth of penetration. Must use laser mask and vacuum because viral DNA has been demonstrated in the smoke plume. Neodymium:yttrium-aluminum-garnet (Nd:YAG) coagulates tissue and causes less plume. Overall success with laser is 88% to 100%. Recurrence may occur in 2 to 3 months. In the United States, most young, sexually active patients who have genital ulcers have genital herpes, syphilis, or chancroid. Although genital herpes is the most prevalent of these diseases, the relative frequency of each differs by geographic area and patient population. Patients with genital ulcers may have more than one disease. Conversely, not all genital ulcers are caused by STIs. Each disease has been associated with an increased risk for HIV infection. A diagnosis based only on the patient’s medical history and physical examination often is inaccurate. Only three ulcer presentations are pathognomonic: 1. A fixed drug eruption is always triggered by the use of one particular medication. 2. A group of vesicles on an erythematous base that does not follow a neural distribution is pathognomonic for herpes simplex infection. 3.
Sexually Transmitted Infections
Introduction
Bacterial urethritis
Gonorrhea
Diagnosis
Treatment of uncomplicated gonococcal urethritis
Recommended regimens
Follow-up
Complications
Chlamydia
Treatment
Recommended regimens
Alternative regimens
Recurrent and persistent infections
Nonchlamydial nongonococcal urethritis
Genital lesions
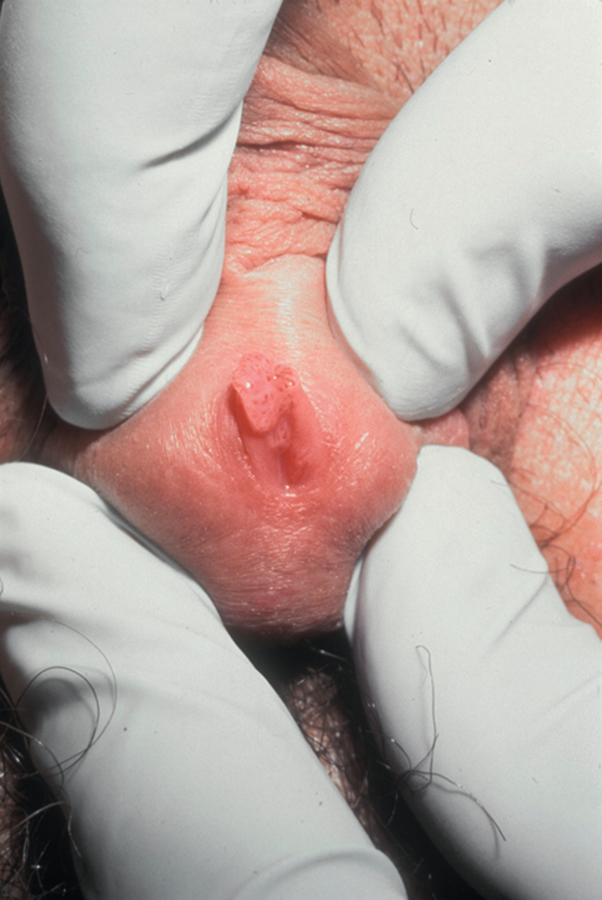
Condyloma acuminata (genital warts)
Treatment
Recommended regimens for external genital warts
Patient-applied
Physician-administered
Surgical removal for very large wart burden
Genital ulcers
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



