Chapter 30 Screening for Esophageal Squamous Cell Carcinoma and Its Precursor Lesions
![]() Video related to this chapter’s topics: Endoscopic Screening for Esophageal Squamous Cell Carcinoma
Video related to this chapter’s topics: Endoscopic Screening for Esophageal Squamous Cell Carcinoma
Introduction
Esophageal cancer is the sixth leading cause of cancer death worldwide.1 In 2002, it was estimated that there were 462,000 new esophageal cancer cases and 386,000 deaths caused by esophageal cancer, only 25,000 fewer deaths than were caused by breast cancer.1 About 80% of esophageal cancer cases occur in developing countries.1 In the United States, esophageal cancer is the ninth leading cause of cancer death; an estimated 16,470 new cases and 14,530 deaths occurred as a result of esophageal cancer in 2009.2
One striking characteristic of esophageal cancer throughout the world is its great geographic variation in incidence, with 10-fold differences reported over distances of a few hundred kilometers.3 Worldwide, the highest risk populations are found in two geographic belts: one in central Asia from the Caspian Sea to north central China and one from eastern to southern Africa (Fig. 30.1).4 Age-adjusted incidence rates over 100 cases per 100,000 inhabitants per year have been reported from some areas in these regions.3,5,6 Populations with intermediate risk are found in southern South America (in southern Brazil, Uruguay, Paraguay, and northern Argentina) and in northwestern France.5 People throughout most of the world are considered to be at low risk, with incidence rates less than 10 per 100,000 inhabitants per year.5 In the Surveillance Epidemiology and End Results (SEER) cancer registries in the United States, the age-adjusted incidence of EC per 100,000 population per year in 2002–2006 was 7.9 in white men, 1.9 in white women, 9.3 in black men, and 3.0 in black women.7 In low-risk countries such as the United States, the male-to-female ratio of cases is usually about 3 : 1 to 4 : 1,7 but in the highest risk populations, this ratio approaches or even falls below 1 : 1.3,6
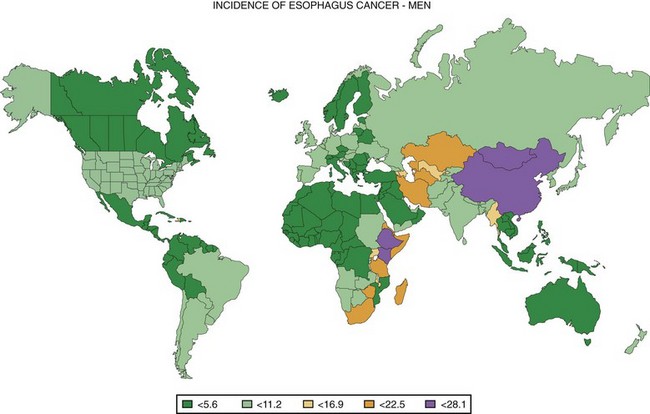
Fig. 30.1 Worldwide incidence of esophageal cancer in men, 2002.
(Data from Ferlay J, Bray F, Pisani P, et al: GLOBOCAN 2002: Cancer incidence, mortality and prevalence worldwide: IARC cancer base No. 5. version 2.0, Lyon, 2004, IARC Press.)
Throughout most of the world, most esophageal cancer cases are esophageal squamous cell carcinomas (ESCCs).5 In Western countries, the incidence of ESCC has been gradually declining, and the incidence of esophageal adenocarcinoma has been rapidly increasing over the last 30 years,5,8 so that now greater than 50% of esophageal cancer cases in the United States are esophageal adenocarcinoma.5,7
In most low-risk countries, cigarette smoking and alcohol consumption are the dominant risk factors for ESCC.9–11 In the United States, greater than 90% of ESCC cases can be attributed to these two exposures alone.12 Additional contributing risk factors include a low dietary intake of fruits and vegetables and factors related to low socioeconomic status.13–16 A few host medical conditions have also been associated with increased risk of ESCC in low-risk populations, including previous or concurrent squamous cell carcinoma of the head and neck region, achalasia, tylosis, caustic esophageal strictures, and Plummer-Vinson syndrome.17
In most high-risk populations, tobacco and alcohol are not major risk factors for ESCC. Tobacco consumption in these groups is typically low, both in terms of the prevalence of smoking and in the amount of tobacco consumed by smokers, and alcohol consumption is even lower.18–20 In addition, in the highest risk areas, where there are nearly as many ESCC cases in women as in men, virtually none of the women smoke or drink.18–20 These high-risk groups may be exposed to some of the major tobacco carcinogens, however, such as polycyclic aromatic hydrocarbons (PAHs), nitrosamines, and acetaldehyde, in other ways. Studies have documented high levels of PAH exposure in Linxian, a county in the high-risk region in north central China21; in northeastern Iran22; and in southern Brazil.23 The source of these exposures is unknown; in Linxian, it may be related to ingestion of ambient soot particles released from heating and cooking with soft coal in unvented stoves,24,25 and in Brazil, it may be caused by drinking the beverage maté.23,26 Nontobacco exposure to nitrosamines and acetaldehyde has also been suggested in Linxian27,28 and in northeastern Iran29 possibly secondary to changes in oral bacteria that accompany poor oral hygiene and tooth loss. Other risk factors reported in high-risk areas include diets low in fruits and vegetables20,30,31; low levels of certain micronutrients, especially selenium and zinc32,33; low socioeconomic status34; exposure to fungal toxins such as fumonisins35; and drinking hot liquids.30,36,37
One of the most consistent risk factors for ESCC in high-risk populations is family history,20,38,39 and preliminary molecular studies support a role for genetic susceptibility in the etiology of ESCC in these areas. Studies have shown high frequencies of loss of heterozygosity (LOH),40 characteristic patterns of gene expression,41 and significant differences in both LOH and gene expression by family history41,42 in tumors from north central China, but no major susceptibility gene for ESCC has yet been identified.
Both cell types of esophageal cancer have a very poor prognosis. In SEER data for 1999–2005, the overall 5-year relative (disease-specific) survival for EC patients was 16.4%.7 This survival rate is improved from 4.7% in 1975–1979,43 but it is still the third lowest survival rate (after pancreas and liver) among major cancers. In developing countries, the 5-year survival rates are usually less than 10%.5
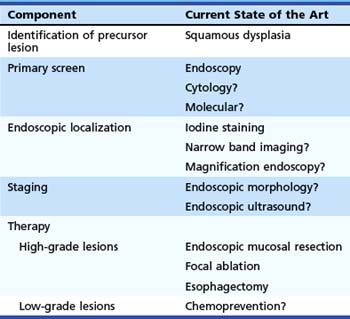
Table 30.1 summarizes these components for ESCC and our understanding of the current techniques available for each component. Techniques that are possible but are not yet established are followed by a question mark. Because the subject of this chapter is screening, we discuss only the first three of these components. Staging and endoscopic therapy are discussed in other chapters of this text.
Table 30.1 Components Needed for Successful Early Detection and Treatment Program for Esophageal Squamous Cell Carcinoma
| Component | Current State of the Art |
|---|---|
| Identification of precursor lesion | Squamous dysplasia |
| Primary screen | Endoscopy |
| Cytology? | |
| Molecular? | |
| Endoscopic localization | Iodine staining |
| Narrow band imaging? | |
| Magnification endoscopy? | |
| Staging | Endoscopic morphology? |
| Endoscopic ultrasound? | |
| Therapy | |
| High-grade lesions | Endoscopic mucosal resection |
| Focal ablation | |
| Esophagectomy | |
| Low-grade lesions | Chemoprevention? |
Precursor Lesions of Esophageal Squamous Cell Carcinoma
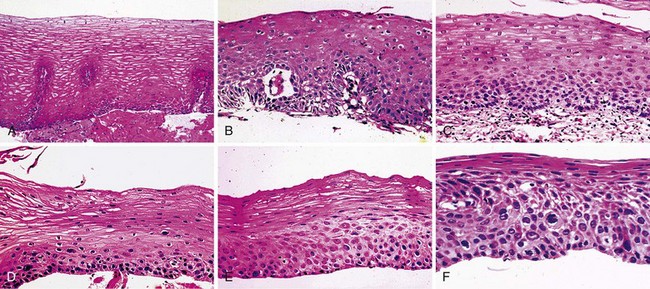
In low-risk countries, squamous dysplasia (including carcinoma in situ) is accepted as the histologic precursor of ESCC because it is the established precursor in other organs lined by squamous epithelium, such as the cervix, and because it is often found adjacent to invasive cancer in esophagectomy specimens.44 In high-risk populations, squamous dysplasia is also accepted as a precursor of ESCC,45–49 but other histologic lesions, such as chronic esophagitis, atrophy, and basal cell hyperplasia, have also been proposed as precursors, based primarily on differences in the prevalence of these lesions in high-risk and low-risk groups.46,49,50 These ecologic comparisons have not always been consistent, however.51 In the only two prospective studies, in which patients who underwent biopsy were followed over time, only squamous dysplasia was significantly associated with the later development of ESCC,45,47,48 and increasing grades of dysplasia were associated with increasing risk.45,48 In the larger of these prospective studies, members of our group followed 682 adults from Linxian who underwent endoscopy without initial evidence of invasive cancer for up to 13.5 years and compared the cumulative incidence and relative risk of developing ESCC among patients with different initial biopsy diagnoses (Fig. 30.2 and Table 30.2).
Table 30.2 Incidence and Relative Risk of Esophageal Squamous Cell Carcinoma (ESCC) during 13.5 Years of Follow-up, by Initial Histology, in an Endoscopy Cohort from Linxian, China
We believe that squamous dysplasia is the only confirmed, clinically relevant precursor lesion of ESCC in high-risk and low-risk populations. More recent consensus conferences and World Health Organization guidelines prefer to use the term intraepithelial neoplasia rather than dysplasia throughout the gastrointestinal (GI) tract, and they prefer to subdivide these lesions into two grades: low-grade intraepithelial neoplasia and high-grade intraepithelial neoplasia.52,53 In the squamous lesions of the esophagus, the guidelines suggest that high-grade intraepithelial neoplasia should include severe dysplasia and carcinoma in situ.52 The above-described data suggest, however, that there are three quite different levels of risk that are identifiable by histologic examination, and both moderate and severe dysplasia need to be targeted for screening and treatment.
Nonendoscopic Screening Techniques
Cytologic Techniques
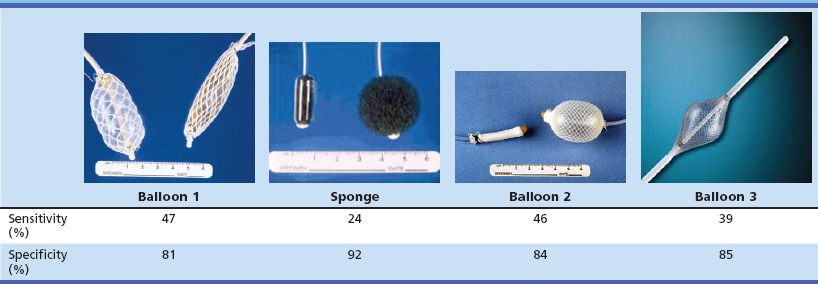
The most common nonendoscopic screening technique for early detection of esophageal cancer is esophageal balloon cytology screening in high-risk populations. Two principal types of cytologic samplers have been used in these screenings: an inflatable balloon sampler first developed in China54–57 and an encapsulated sponge sampler first developed in Japan (Fig. 30.3).58–61 In the balloon technique, a deflated balloon covered by a cloth net or rubber ribbing is swallowed into the stomach, inflated, and withdrawn, collecting exfoliated cells and scraping the mucosal surface of the esophagus. At the upper esophageal sphincter, the balloon is deflated and removed. In the sponge technique, a polyurethane mesh is compressed inside a gelatin capsule and attached to a string or a thin plastic stylet. The capsule is swallowed into the stomach, where the gelatin dissolves, and the mesh expands. The mesh is pulled up the esophagus by the string, collecting exfoliated and scraped mucosal cells. In both methods, the collected cells are processed and stained for cytology and read for cellular abnormalities. Several studies of both of these methods have reported high sensitivities for detecting ESCC in symptomatic patients, but there are few data on their accuracy for detecting squamous dysplasia or for detecting ESCC in asymptomatic individuals, who would be the target group for any population screening effort.
To investigate further the potential of nonendoscopic esophageal cytology for screening asymptomatic high-risk individuals, we performed two studies in Linxian to evaluate the screening characteristics of two commonly used Chinese balloons, a new American balloon, and an American-made encapsulated sponge. In this study, asymptomatic adults were examined by the cytology samplers, followed by Lugol’s iodine chromoendoscopy of all participants. The cytology slides were read using the Bethesda System, the standard Western cytologic criteria for diagnosing cervical and vaginal smears, and the cytologic diagnoses were compared with the “gold standard” biopsy diagnoses. In the first study,62 439 patients were examined by both a Chinese balloon and the encapsulated sponge, in random order, and in the second study,63 359 patients were examined by the second Chinese balloon, and 381 patients were examined by the American balloon. The results of these studies were not encouraging: None of the samplers achieved a sensitivity of 50% for identifying biopsy-proven squamous dysplasia or cancer (Table 30.3). The most important problem with this screening technique appears to be two kinds of sampling error: blind sampling of a large organ and missing small lesions and morphologic evaluation of only a small percentage of the collected cells.
Table 30.3 Screening Characteristics of Esophageal Balloon Cytology Samplers for Identifying Patients with Biopsy-Proven Esophageal Squamous Dysplasia in Linxian, China
Molecular Techniques
Blood or one of its components would be an ideal clinical sample for primary screening purposes. Possible serologic approaches to screening include looking for tumor-specific DNA, RNA, or proteins that are secreted into the circulation or are released during tumor cell death and looking for autoantibodies produced by the host in response to tumor antigens. A few authors have looked for tumor-specific hypermethylated genes in the serum or plasma of patients with ESCC and have found them to be present in a few cases.64,65 Hibi and colleagues64 found hypermethylated p16 in tumor tissue in 31 (82%) of 38 ESCCs and found this same marker in the serum of 7 (23%) of the 31 patients with positive tumors. Kawakami and associates65 found hypermethylated APC in tumor tissue from 16 (50%) of 32 ESCCs and in the corresponding serum from 2 (12%) of the 16 tumor-positive patients. In the latter study, detection of hypermethylated APC in the plasma of esophageal adenocarcinomas was significantly associated with tumor stage (1 of 26 [4%] positive in stage I-II tumors; 12 of 26 [46%] positive in stage III-IV tumors); this should probably also be true of ESCCs. Although the proportion of tumors showing some hypermethylation increases when multiple genes are evaluated,66 it still seems unlikely that many intraepithelial precursor lesions or stage I ESCCs would shed altered DNA into the serum or plasma that can be detected by such evaluations. Preliminary serologic studies looking for tumor-associated RNA67 and proteins68 have also been performed. Another possible serologic screening approach that has shown promise for detection of early squamous cell carcinomas of the lung and the head and neck region is identification of host autoantibodies generated against tumor-specific antigens.69–71
Stool is another clinical sample that could potentially contain information about the esophagus and could be collected noninvasively. Several authors have shown that neoplasia-specific DNA mutations can be detected in stool from patients with colorectal adenomas and adenocarcinomas,72,73 and the sensitivity of these assays can be increased by testing panels of markers73a and by employing more sensitive new technologies.74 There are also two reports of detection of other, more proximal aerodigestive malignancies, including esophageal carcinomas, by measuring high-molecular-weight or “long” DNA (DNA from nonapoptotic cells, which are more commonly sloughed from neoplasms)75 or tumor-specific DNA mutations76 in stool. Detection of esophageal precursor lesions or early invasive ESCCs by these methods may be unlikely, but it should still be evaluated.
At least for the near future, nonendoscopic molecular screening for early ESCC and its precursor lesions may still need to depend on evaluation of esophageal cell samples. As discussed previously, current morphology-based cytologic techniques are not sufficiently sensitive to find patients with focal squamous dysplasia, but molecular techniques may improve this sensitivity, especially DNA changes that can be amplified. One study reported promoter methylation findings for eight genes in esophageal balloon cytology samples from 147 patients with endoscopic biopsy diagnoses ranging from normal to severe squamous dysplasia and showed a 50% sensitivity and 68% specificity for identifying patients with high-grade dysplasia.77 The most promising possibility may be the detection of molecular changes that have undergone clonal expansion and affect large areas of the squamous mucosa, similar to the “field effects” previously documented for p16 and p53 lesions in Barrett’s esophagus.78,79 If such field-wide molecular abnormalities are identified that reliably precede or accompany squamous dysplasia, a simple, imperfect sampler that is acceptable to patients, such as an encapsulated sponge, may be able to identify accurately or rule out the presence of an abnormal field, which may be sufficient to triage patients appropriately to endoscopy.
Endoscopic Screening Techniques
Various different endoscopes have been used to screen for early ESCC and its precursor lesions. These endoscopes may differ in size, resolution, and magnification characteristics. Ultrathin endoscopes measuring less than 6 mm (with a biopsy channel) may be used. A trial with a 3.1-mm stand-alone battery-powered fiberoptic esophagoscope (which did not have a biopsy channel) showed that it was feasible and accurate in detecting esophageal pathology.80 Typically, instruments with diameters of 9 mm are employed. Standard resolution and magnification instruments suffice, but advantages of higher resolution and magnification have been described.81 Conventional videoendoscopes are equipped with CCD chips of 100k to 300k pixels, meaning that each image is built up from 100,000 to 300,000 individual pixels. This technical feature, pixel density, determines the resolution. Newer instruments with CCDs having 600,000 to 1 million pixels are now commercially available and are referred to as high-resolution instruments. The feature of high resolution is distinct from high magnification, which can be accomplished by either optical magnification or electronic magnification. With optical magnification, a mechanical system that can move a lens at the tip of the endoscope along the longitudinal axis of the endoscope creates the ability for the endoscopist to adjust the focal distance of the device to allow for close viewing of mucosal details. Depending on which endoscope and which optical magnification system is used, the image can be enlarged 75 to 115 times. With optical magnification, image resolution can be preserved during the magnification process. With an electronic magnification technique, the actual magnification is performed by the videoprocessor. An area in the center of the endoscopic images magnifies only the pixels in the target area, which are used to generate the enlarged image. It is often more difficult to preserve image resolution in this method. Features of high resolution and high magnification can be combined. Most of the published literature regarding endoscopic screening describes the use of standard endoscopes.
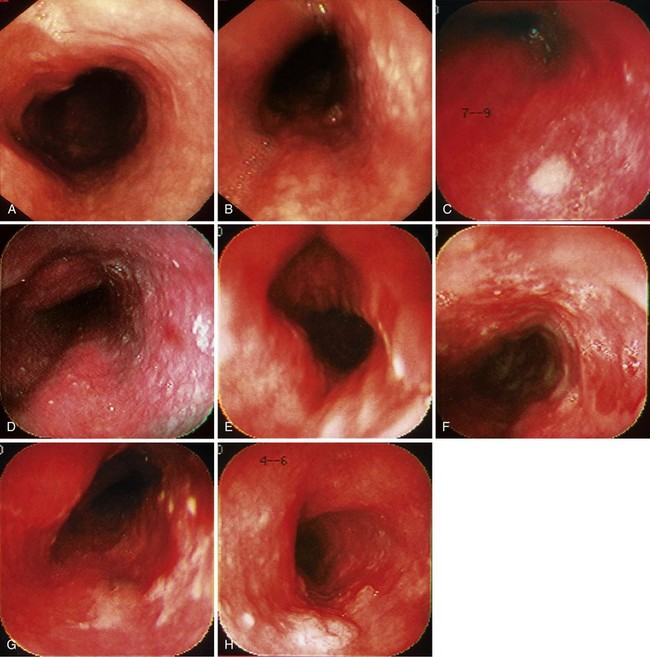
When screening is performed endoscopically, visual inspection to identify pathology is the first step. In the absence of staining, dysplasia may appear as normal mucosa, irregular mucosa, a small white patch, a focal red area, an erosion, or a plaque. Early ESCC is usually seen as an erosion, a plaque, or a nodule (Fig. 30.4).82
Chromoendoscopy
Chromoendoscopy has been shown to assist with definition of early precancerous and cancerous lesions in numerous patients. When chromoendoscopy is used, Lugol’s iodine is generally employed for staining. The first description of iodine staining was by Schiller in 1933,83 and it was used in the uterine cervix. The first reports using iodine as a supplement to esophagoscopy were by Brodmerkel,84 Nothmann and colleagues,85 and Voegeli.86
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree