Fig. 5.1
Specificity of 89Zr-trastuzumab for HER2-positive tumors. Coronal 89Zr-trastuzumab, 18F-FDG, and 18F-FLT PET images of athymic nude mice bearing subcutaneous HER2-positive NCI-N87 ( left) and HER2-negative MKN-74 ( right) tumors are shown. +ve = positive; −ve = negative. This research was originally published in JNM. (Janjigian YY, Viola-Villegas N, Holland JP, et al. [57]; images on the right: © by the Society of Nuclear Medicine and Molecular Imaging, Inc.)
Patient-Derived Xenografts
Individual esophagogastric cancer subtypes have heterogenous tumor characteristics and clinic outcomes, making this malignancy a complex disease to treat. Cell culture lines and even mouse xenografts of human tumor cell lines have had variable predictive power in the translation of cancer therapies into clinical setting [68]. These models often fail to reproduce the complexities of the tumor microenvironment and the interaction between the tumor cells and the immune system, which are integral components to tumor proliferation and metastasis [69].
Tumor graft models or patient-derived xenografts (PDXs) are being studied as an alternative, more clinically predictive model of human malignancies. PDXs are based on the transfer of primary tumors directly from the patient into an immunodeficient mouse. The tumors can be implanted either heterotopically or orthotopically. Heterotopic PDX model involves implanting tumors into the subcutaneous tissue of the mouse. Orthotopic models involve direct implantation of the tumor into a specific mouse organ. The heterotopic method allows for easier cell transfer and precise monitoring of tumor growth. The orthotopic models, while more technically challenging, are considered to more accurately mimic the human tumors [70]. Limitations of using PDX models for research include the higher cost and more specialized maintenance compared to cultured cell lines. Furthermore, PDX models can require long latency periods after engraftment and variable engraftment rates between 23 and 75 % depending on the tumor type [69, 71].
PDX models are actively being studied in esophagogastric cancers. Janjigian and colleagues at MSKCC have established both heterotopic and orthotopic PDX models using nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse (Fig. 5.2). The established PDXs include HER2-positive trastuzumab refractory models, MET+ models, and signet ring gastric model with germ line CDH1 mutation. Tumor engraftment rates of 46 % for orthotopic tumors and 26 % for heterotopic implants were reported [72]. PDX models are a promising platform to further validate differences in tumor biology and guide the design of clinical trials. Further, molecular profiling and therapeutic experiments with the PDX models are underway to identify distinct molecular signatures predictive of response to these agents.
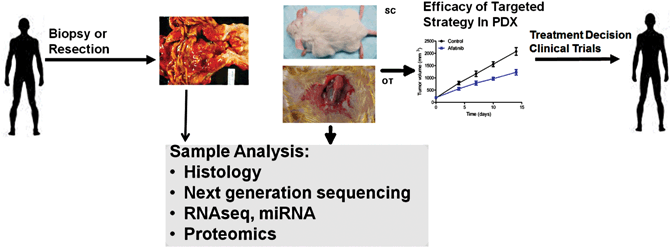

Fig. 5.2
MSKCC Patient-Derived Xenograft ( PDX) Program Schema: esophagogastric cancer models implemented to bring targeted agents to the clinic
Genomic Sequencing
Next generation sequencing (NGS) has allowed for cheaper and faster sequencing compared to traditional Sanger sequencing. The Cancer Genome Atlas (TCGA) is an ongoing research program supported by the National Cancer Institute and National Human Genome Research Institute at the National Institutes of Health. The TCGA researchers will identify the genomic changes in more than 20 different types of human cancer, including gastric and esophageal cancers. The genomic sequencing data will be available to the research community and allow for a more comprehensive understanding of the acquired genetic, genomic, and epigenetic alterations in cancer cells that can be translated into clinical and therapeutic advances.
The integrated esophagogastric TCGA data provide insight in the tumorigenesis of gastric cancers and identify further targetable mutations, beyond HER2. Whole exome and genome sequencing of esophageal adenocarcinoma tumors and normal pairs identified 26 significantly mutated genes. The sequencing identified novel mutated genes not previously implicated in this disease, including mutations in chromatin modifying factors and candidate contributors: SPG20, TLR4, ELMO1, and DOCK2 [73]. The esophagogastric TCGA identified four distinct subsets of the disease: (1) Epstein Barr Virus (EBV) tumors with marked methylation, PIK3CA mutations, PD-L1/2 amplification, (2) Tumors with Microsatellite instability (MSI) and frequent activating mutations, (3) chromosomally instable (CIN) tumors with frequent oncogenic amplifications, and (4) chromosomally stable/diffuse type tumors with novel mutations of RHOA (ras homolog gene family, member A). RHOA encodes a small guanosine-5′-triphosphatase (GTPase) that displays potent oncogenic activity when overexpressed. Recent TCGA sequencing data on diffuse-type gastric carcinoma revealed newly identified recurrent RHOA hotspot mutations in diffuse-type gastric cancers, which were not seen in intestinal-type tumors [74, 75]. The presence of RHOA mutations was associated with tumors located in the cardia, poorer tumor differentiation, and less likely to be associated with TP53 mutations. Further detailed mechanistic and translational studies are ongoing [74].
At Memorial Sloan Kettering Cancer Center, NGS using the integrated mutation profiling of actionable cancer targets (IMPACT) assay is being performed to identify previously unrecognized biomarkers of drug sensitivity and resistance. The IMPACT assay is capable of identifying point mutations, small insertion/deletion events (indels), and large gene level and intragenic copy number aberrations in 275 cancer-associated genes. In the ongoing phase II study of afatinib in metastatic HER2-positive, trastuzumab refractory cancer, pre- and posttreatment biopsies are being collected in all patients, allowing for a unique opportunity to define the prevalence of p95-HER2 and other genetic aberrations that have been associated with trastuzumab resistance in preclinical models [57].
Genomic sequencing technology will allow for the comprehensive profiling of tumor specimens and holds the potential to guide cancer treatment. Efforts are ongoing at institutions worldwide to correlate the genetic and molecular information of the genomic sequencing data with clinical data to guide individualized therapies and diagnostic tools .
Conclusions
The majority of patients with gastric cancer present with advanced disease, which is incurable. Molecularly targeted therapies , such as those targeting HER2, are anticipated to improve the current status of systemic treatment beyond conventional cytotoxic therapy. Trastuzumab in combination with chemotherapy in patients is the first molecular agent in metastatic HER2-positive gastric and gastroesophageal to result in improvements in response rates, time to progression and survival. Trastuzumab is now being investigated in the neoadjuvant and adjuvant setting. Unfortunately, as with breast cancer, many esophagogastric patients will develop resistance to trastuzumab. Several promising therapeutic agents are currently under investigation as monotherapy and in combination with chemotherapy in the first and second line setting. New avenues of research into mechanisms of resistance and technology to better diagnose and treat HER2 gastric cancer are being actively studied.
References
1.
3.
4.
5.
Bang Y, Chung H, Xu J, et al. Pathological features of advanced gastric cancer (GC): relationship to human epidermal growth factor receptor 2 (HER2) positivity in the global screening programme of the ToGA trial. J Clin Oncol. 2009;27:15 (supp; abst 4556).CrossRef
6.
7.
8.
Rüschoff J, Dietel M, Baretton G, et al. HER2 diagnostics in gastric cancer—guideline validation and development of standardized immunohistochemical testing. Virchows Archiv. 2010;457:299–307.PubMedCentralPubMedCrossRef
9.
National Comprehensive Cancer Network (NCCN). Esophageal and esophagogastric junction cancers. Version 2.2013. http://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. Accessed 11 March 2014.
10.
11.
13.
Terashima M, Ochiai A, Kitada K, et al. Impact of human epidermal growth factor (EGFR) and ERBB2 (HER2) expressions on survival in patients with stage II/III gastric cancer, enrolled in the ACTS-GC study. J Clin Oncol. 2011;29 (suppl; abstr 4013).
14.
Yoon HH, Shi Q, Sukov WR, et al. HER2 expression/amplification: frequency, clinicopathologic features, and prognosis in 713 patients with esophageal adenocarcinoma (EAC). J Clin Oncol. 2011;29 (Suppl). (Abstr 4012).
15.
Grabsch H, Sivakumar S, Gray S, et al. HER2 expression in gastric cancer: rare, heterogeneous and of no prognostic value—conclusions from 924 cases of two independent series. Cell Oncol. 2010;32:57–65.PubMed
16.
Janjigian YY, Werner D, Pauligk C, et al. Prognostic significance of human epidermal growth factor-2 (HER2) in advanced gastric cancer: a US and European international collaborative analysis. Ann Oncol. 2012;23(10):2656–62.PubMedCrossRef
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







