Fig. 5.1
Model of a parietal cell showing stimulatory receptors on its basal–lateral plasma membrane and their second messengers. AC adenylate cyclase, Ach acetylcholine, Ca 2+ calcium ion, cAMP cyclic adenosine monophosphate, CCK cholecystokinin, ECL enterochrommafin like, G gastrin, H histamine, H + , K + -ATPase hydrogen, potassium-ATPase (proton pump), M muscarinic. (Reprinted by permission from Macmillan Publishers Ltd.: Feldman MJ 2013)
Inhibition of either of the acetylcholine, histamine, or gastrin receptors will decrease acid production to a degree. Importantly, inhibition of the H+/K+-ATPase enzyme acts upon the final common pathway, and is the reason for the selective superiority of PPIs. However, by interfering at different points along the pathway of acid secretion, both H2RAs and PPIs inhibit gastric acid secretion and raise intragastric pH levels.
H2-Receptor Antagonists
Prior to the development of PPIs , H2RAs were the primary class of medication prescribed for treating GERD. There are four H2RAs currently available on the market: cimetidine, famotidine, nizatidine, and ranitidine (Table 5.1) [2–5]. The first medication, cimetidine, was developed in the 1960s, first marketed in 1976 and became one of the first “blockbuster drugs.” H2RAs became available as over-the-counter (OTC) medications in 1995 and are still widely used today, especially in patients who are not able to take PPIs or in patients who take it in combination with PPI therapy. As a class of medication, H2RAs competitively antagonize histamine at the level of the parietal cell’s H2 receptor, and their effectiveness comes as a sole result of inhibiting acid secretion. They do not effect LES pressure, decrease TLESRs , or augment either esophageal or gastric emptying. In general, the efficacy of gastric acid inhibition is best at night, when the medication is taken before dinner or at bedtime.
Table 5.1
Currently available H2RAs
Generic name | Brand name | Oral dosage strengths | Half-life (h) | Costa |
|---|---|---|---|---|
Cimetidine [2] | Tagamet | Tablets: 200 and 400 mg | 2 | Strength: 200 mg Quantity: 30 tablets OTC cost: US$ 13.99 |
Famotidine [3] | Pepcid | Tablets: 10, 20, and 40 mg Oral solution: 40 mg/5 mL | 2.5–3.5 | Strength: 20 mg Quantity: 25 tablets OTC Cost: US$ 12.99 |
Nizatidine [4] | Axid | Capsules: 150 and 300 mg Oral solution: 15 mg/mL | 1–2 | Strength: 150 mg Quantity: 30 tablets Cost: US$ 69.99 |
Ranitidine [5] | Zantac | Tablets: 75, 150, and 300 mg Oral solution: 15 mg/mL | 2.5–3 | Strength: 75 mg Quantity: 30 tablets OTC cost: US$ 10.99 |
Among patients on H2RA therapy, symptom relief and endoscopic improvement of esophagitis varies significantly, ranging from 32 to 82 % and 0 to 82 %, respectively [6]. One review showed that complete healing was seen endoscopically in only 27–45 % of patients, and this was primarily in those patients with milder degrees of esophagitis [7]. Increasing the strength or the frequency of H2RA dosing up to two to four times per day may increase esophageal mucosal healing. One large study of 696 patients with GERD showed that ranitidine 150 mg four times per day produced significantly higher mucosal healing rates at 12 weeks than ranitidine 150 mg twice per day or cimetidine 800 mg twice per day (77 vs. 71 and 68 %, respectively) [8]. In another study of 474 patients with erosive esophagitis comparing famotidine 20 mg twice per day versus 40 mg twice per day, relief of symptoms was significant in all patients at 6 and 12 weeks, but did not differ between treatment groups. Endoscopic healing was significantly better in the famotidine 40 mg twice per day group compared with 20 mg twice per day at both week 6 (58 vs. 43 %) and week 12 (76 vs. 67 %) [9]. Overall, the wide variability in the literature, especially with regard to symptom and endoscopic improvement, is likely due to inconsistency in symptom end points and variability in interpreting endoscopic baselines .
Side Effects
As a drug class, H2RAs are well tolerated, have few side effects, and are generally safe to use. The most common side effects are gastrointestinal, including nausea, vomiting, abdominal pain or bloating, diarrhea, and constipation. Other side effects include headaches, dizziness, and rashes. H2RAs are metabolized through the liver cytochrome P450 pathway. This raises the possibility of drug–drug interactions, especially with other agents that are also metabolized through the same pathway. This is particularly the case with cimetidine, the first H2RA. Serum concentrations of several drugs are altered following administration of cimetidine including warfarin, theophylline, phenytoin, lidocaine, procainamide, tramadol, and beta-blockers. Cimetidine is also a competitive antagonist of the dihydrotestosterone (DHT) receptor. This was shown to lead to galactorrhea in women and gynecomastia in men. The more recently developed H2RAs are not as potent inhibitors of the cytochrome P450 pathway and appear less likely to significantly alter the metabolism of other agents. It does not appear that H2RAs affect the serum concentration of clopidogrel.
Proton Pump Inhibitors (PPIs)
PPIs are the most widely used class of medications for treating patients with GERD and are the most effective agents. There are currently seven PPIs available on the market (Table 5.2) [10–16]. Five are delayed release medications: omeprazole, esomeprazole, pantoprazole, lansoprazole, and rebeprazole. Another is omeprazole immediate release-sodium bicarbonate, which is a combination of non-enteric-coated omeprazole with sodium bicarbonate (OME-IR). The last is dexlansoprazole, which is the R-enantiomer of lansoprazole and utilizes a dual-release technology with two types of enteric-coated granules that dissolve at different pHs. This drug first dissolves in the duodenum and produces a peak plasma level approximately 1 h after administration. The second component dissolves in the distal small intestine and produces a second peak approximately 4 h later [17]. Four PPIs are available OTC: omeprazole, omeprazole with sodium bicarbonate, esomeprazole, and lansoprazole.
Table 5.2
Currently available PPIs
Generic name | Brand name | Oral dosage strengths | Half-life (h) | Costa |
|---|---|---|---|---|
Over the counter (OTC) | ||||
Omeprazole [10] | Prilosec | Delayed-release capsules: 10, 20, and 40 mg Delayed-release oral suspension: 2.5 mg, 10 mg | 0.5–1 | Strength: 20 mg Quantity: 28 tablets OTC cost: US$ 21.99 |
Omeprazole and Sodium bicarbonate [11] | Zegerid | Capsules: 20 mg omeprazole and 1100 mg sodium bicarbonate 40 mg omeprazole and 1100 mg sodium bicarbonate Powder for oral suspension: 20 mg omeprazole and 1680 mg sodium bicarbonate 40 mg omeprazole and 1680 mg sodium bicarbonate | 1 | Strength: 20/1100 mg Quantity: 14 capsules OTC cost: US$ 12.99 |
Esomeprazole magnesium [12] | Nexium | Delayed-release capsules: 20 and 40 mg Delayed-release oral suspension: 2.5, 5, 10, 20, and 40 mg | 1–1.5 | Strength: 20 mg Quantity: 28 capsules OTC cost: US$ 21.99 |
Lansoprazole [13] | Prevacid | Capsules and tablets: 15 and 30 mg | 1.5 | Strength: 15 mg Quantity: 28 tablets OTC cost: US$ 21.99 |
Prescription medications | ||||
Rebeprazole sodium [14] | Aciphex | Delayed-release tablets: 20 mg Delayed-release capsules: 5 and 10 mg | 1–2 | Strength: 20 mg Quantity: 30 tablets Cost: US$ 306.99 |
Pantoprazole sodium [15] | Protonix | Delayed-release tablets: 20 and 40 mg Delayed-release oral suspension: 40 mg | 1 | Strength: 20 mg Quantity: 30 tablets Cost: US$ 119.99 |
Dexlansoprazole [16] | Dexilant | Delayed-release capsules: 30 and 60 mg | First Peak at 1–2 Second Peak at 4–5 T1/2 = 1–2 | Strength: 30 mg Quantity: 30 tablets Cost: US$ 264.99 |
PPIs are all highly selective and concentrate in the strongly acidic environment of the secretory canaliculi of the parietal cells. Once the PPI is present in the acidic environment, the inactive benzimidazole converts to a cationic sulfonamide, which then binds to the H+/K+-ATPase preventing gastric acid production [18, 19]. However, it is important to recognize that gastric acid inhibition following PPI administration is delayed because these drugs need time to build up in the secretory canaliculi and inhibit the H+/K+-ATPases. Therefore, to achieve maximal effect, it is recommended to take PPIs 30 min before the first meal of the day, and not with the meal. Given that PPIs bind to H+/K+-ATPases irreversibly, new proton pump enzymes must be produced for gastric acid secretion to continue. PPIs block approximately 70–80 % of active pumps, as new H+/K+-ATPases are continuously being produced. As a result, a single dose of a PPI does not prevent all acid secretion. When a PPI is taken twice daily, more H+/K+-ATPases become irreversibly bound to the drug, thus the effect on gastric acid inhibition is potentiated. Given the dual-release technology of dexlansoprazole, medication administration prior to meals is not as necessary as with the delayed-release PPIs.
pH Control
PPIs demonstrate superior pH control over H2RAs over a 24-h period. While omeprazole, OME-IR, rabeprazole, pantoprazole, and lansoprazole all provide a similar degree of intragastric pH control (11–13 h with pH > 4), esomeprazole at 40 mg daily dosing does provide a slightly longer duration of control (Fig. 5.2) [20, 21]. The newest PPI, dual-release dexlansoprazole, has been shown to maintain pH > 4 for up to 17 h with once-daily administration [16].
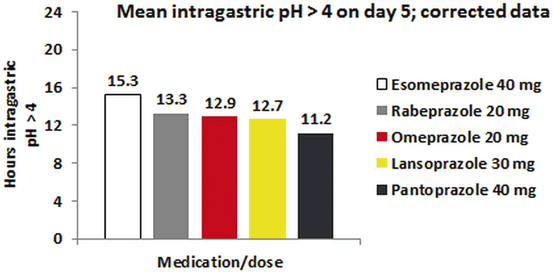

Fig. 5.2
Percentage time intragastric pH above 4 for five delayed-release proton pump inhibitors given once daily before breakfast. (Adapted with permission from Richter JE, Castell D 2012)
Healing of Erosive Esophagitis and Control of Symptoms
While PPIs may not lead to complete symptom relief in all patients, they are superior to H2RAs in their capacity to improve symptoms [22] . In addition, compared with H2RAs, PPIs have shown superior healing rates in patients with erosive esophagitis [23]. A large meta-analysis of 43 articles in 1997 showed superior healing of all grades of erosive esophagitis and heartburn relief when using PPIs, as compared with H2RAs, sucralfate, or placebo [22]. The mean overall healing percentage irrespective of drug dose or treatment duration ( ≤ 12 weeks) was the highest with PPIs (83.6± 11.4 %) versus H2RAs (51.9 ± 17.1 %), sucralfate (39.2 ± 22.4 %), or placebo (28.2 ± 15.6 %). The mean heartburn-free proportion of patients was highest with PPIs (77.4 ± 10.4 %) versus H2RAs (47.6 ± 15.5 %), and PPIs showed a significantly faster healing rate (11.7 %/week) versus H2RAs (5.9 %/week) and placebo (2.9 %/week).
While all PPIs have similar healing rates of erosive esophagitis after 8 weeks of treatment, esomeprazole 40 mg has shown a small advantage when compared with omeprazole 20 mg, pantoprazole 40 mg, and lansoprazole 30 mg [24–26]. Esomeprazole’s advantage is mostly seen with LA grades C and D esophagitis. Another large meta-analysis in 2006 compared rates of esophagitis healing and symptom relief with esomeprazole versus alternative PPIs (except OME-IR and dexlansoprazole) [27]. The analysis included 10 studies and 15,136 patients. At 8 weeks, there was a 5 % (relative risk, RR, 1.05; 95 % CI 1.02–1.08) relative increase in the probability of erosive esophagitis healing with esomeprazole, which led to an absolute risk reduction of 4 % and a number needed to treat (NNT) of 25. The calculated NNTs by LA grades A through D were 50, 33, 14, and 8, respectively. Esomeprazole also led to an 8 % (RR, 1.08; 95 % CI 1.05–1.11) relative increase in the probability of GERD symptom relief at 4 weeks .
In a comparative trial of dexlansoprazole 60 or 90 mg daily with lansoprazole 30 mg daily for 8 weeks, dexlansoprazole achieved non-inferiority to lansoprazole [28]. Dexlansoprazole achieved healing rates of 92–95 % of patients in individual studies versus 86–92 % for lansoprazole, but the difference was not statistically significant (p > 0.025). However, in an integrated analysis of healing in patients with moderate-to-severe erosive esophagitis (LA grades C and D), dexlansoprazole was superior to lansoprazole at healing severe disease. Figure 5.3 shows a summary of healing rates at 8 weeks among various PPIs [21, 24–26, 28, 29].
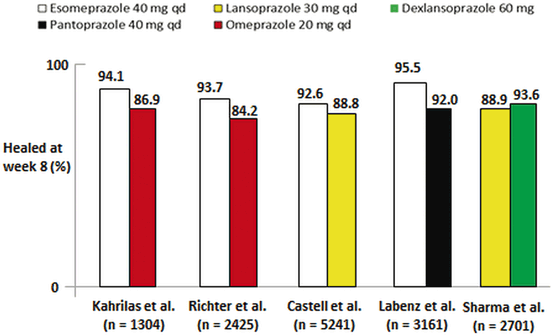

Fig. 5.3
Healing of erosive esophagitis at 8 weeks with various delayed-release proton pump inhibitors. (Adapted with permission from Richter JE, Castell D 2012)
General Treatment Approach
Most delayed-release PPIs are taken once daily and administered in the morning. With its dual-release technology, dexlansoprazole is approved for dosing without regard to the timing of food intake. The rationale behind morning dosing for delayed-release PPIs stems from an intragastric pH study which assessed the effects of different dosing schedules on pH [30]. This crossover study treated 21 healthy patients with either omeprazole 20 mg or lansoprazole 30 mg daily for 7 days, with the dose given 15–30 min before breakfast, and also on an empty stomach without any food until lunch. Intragastric pH was monitored from 8:00 a.m. to 4:00 p.m. to determine the percentage time gastric pH was below 4.0. Administering the PPI before breakfast led to significantly improved daytime intragastric pH control compared to taking the PPI on an empty stomach and then not eating for several hours. Backed by this data, along with clinical experience, we suggest that PPIs should be taken 30 min before a meal, ideally breakfast, and that this once-daily dosing regimen leads to improvement in symptoms in the majority of patients.
While once-daily PPI dosing is usually very effective, some patients require an increase in dosage, usually given just before the evening meal. This may be due to the presence of persistent GERD symptoms, Barrett’s esophagus, or extraesophageal symptoms. When this is the case, increasing the PPI to twice daily does lead to increased pH control. Maintenance PPI therapy should be considered in patients who have recurrent GERD symptoms once a PPI is discontinued, and in patients with complications such as erosive esophagitis or Barrett’s esophagus [31]. When patients require long-term PPI therapy, the medication should be taken at the lowest effective dose.
Switching a patient from one PPI to another PPI is very common in clinical practice. However, there is very little data to support this approach. One multicenter, randomized, double-blind trial evaluated patients with persistent heartburn while on lansoprazole 30 mg daily [32]. Patients were randomized to 8 weeks of lansoprazole 30 mg twice daily or esomperazole 40 mg daily. The primary end point was the percentage of heartburn-free days from day 8 to the end of treatment. The data showed that both treatment arms were equally effective for heartburn-free days (55 % esomeprazole vs. 58 % lansoprazole), symptom score improvement (for heartburn, acid regurgitation, and epigastric pain) , and rescue antacid use (0.4 tablets/day in the esomeprazole group vs. 0.5 tablets/day in the lansoprazole group). The authors concluded that switching to a different PPI was just as effective as increasing patients’ PPI to twice daily. Currently, there is not any data that supports switching to a different PPI more than once.
Nocturnal Reflux
Many GERD patients suffer from nocturnal symptoms, which is likely an underappreciated problem. While sleeping, the body’s natural defense mechanisms against GERD, such as saliva production and peristalsis, are significantly reduced. Nocturnal reflux can significantly impact quality of life and lead to sleep disturbances. Critical to symptom control is maintaining a gastric pH > 4. However, intragastric pH monitoring studies show that despite being on twice-daily PPI therapy, overnight pH can drop to less than 4 for over an hour [33]. This is called nocturnal acid breakthrough (NAB).
Patients with NAB have several treatment options: (1) single-dose PPI can be administered before the evening meal, (2) patients can be placed on a PPI before breakfast and OME-IR or an H2RA at bedtime, or (3) patients can be placed on twice-daily PPI plus an H2RA at bedtime. One study of 49 patients found that bedtime administration of OME-IR had superior overnight intragastric pH control compared with lansoprazole and esomeprazole [34]. H2RAs are used most commonly to optimize overnight pH control when given at bedtime as an adjunct to PPI therapy. One small study of 12 volunteers found that omeprazole 20 mg twice daily plus ranitidine (150 mg or 300 mg) at bedtime provided superior overnight pH control compared with omeprazole 20 mg twice daily plus an additional omeprazole dose at bedtime [35]. Another study of 105 GERD patients on PPI twice daily (60 patients) or PPI twice daily plus an H2RA at bedtime (45 patients) showed that the median percentage time that gastric pH remained > 4 was 51 % in the twice-daily PPI group compared with 96 % in the twice-daily PPI plus bedtime H2RA group [36]. This contrasts with another study of 22 patients (13 with GERD and 9 controls) which evaluated pH control after each of four treatment regimens: (1) omeprazole 20 mg twice daily for 2 weeks, (2) omeprazole 20 mg twice daily plus ranitidine 300 mg at bedtime for 4 weeks, (3) omeprazole 20 mg before breakfast and at bedtime for 2 weeks, and (4) omeprazole 20 mg every 8 h for 2 weeks [37]. Results showed that the treatment regimens resulted in NAB elimination in 9–41 % of patients. However, no single treatment regimen resulted in more significant NAB control than the others and there were not any differences in percentage time that pH was < 4 for any treatment regimen .
There is concern over H2RA tolerance, that is, the potential that H2RAs may lose their effect following prolonged use. One study of 20 GERD patients and 23 healthy volunteers obtained baseline pH testing and then administered 2 weeks of omeprazole 20 mg twice daily before meals [38]. pH testing was then repeated. Subjects next received 4 weeks of PPI plus ranitidine 300 mg at bedtime, and pH testing was obtained on days 1, 7, and 28. Results showed that combination PPI and H2RA therapy reduced NAB only with the introduction of therapy. No difference in acid suppression between the twice-daily PPI and twice-daily PPI plus H2RA groups was seen following 1 week of combination therapy. In the majority of patients following 1 month of H2RA therapy, gastric acidity returned to pre-H2RA levels.
While many patients may develop tolerance to H2RAs, clinical experience has shown that some patients have a sustained response. The most recent American College of Gastroenterology (ACG) guidelines state that a bedtime H2RA can be added to daytime PPI therapy in patients with evidence of nighttime reflux [31]. To reduce the chance of drug tolerance, as-needed use of an H2RA at night might be more practical if the patient eats late or has an unusually large evening meal .
Special Clinical Situations
Nonerosive Reflux Disease
The majority of GERD patients have a normal endoscopy and, therefore, NERD. Among patients who experience symptoms of heartburn with NERD, PPI therapy has been shown to be superior to H2RAs and prokinetics . In a large Cochrane systematic review of 32 trials, the RR for heartburn remission in placebo-controlled trials for PPIs was 0.37 (two trials, 95 % CI 0.32–0.44), for H2RAs was 0.77 (two trials, 95 % CI 0.6–0.99), and for prokinetics was 0.86 (one trial, 95 % CI 0.73–1.01) [39]. In a direct comparison of PPIs and H2RAs, PPIs were more effective at achieving heartburn remission (seven trials, RR 0.66, 95 % CI 0.6–0.73). In the treatment of endoscopy-negative reflux disease, the RR for heartburn remission for PPI versus placebo was 0.71 (ten trials, 95 % CI 0.65–0.78) and for H2RA versus placebo was 0.84 (two trials, 95 % CI 0.74–0.95). The RR for PPI versus H2RA was 0.78 (three trials, 95 % CI 0.62–0.97). The authors’ conclusion was that PPIs are more effective than H2RAs in relieving heartburn in patients with endoscopy-negative reflux disease, although the magnitude of benefit was greater for those treated empirically [39].
Interestingly, however, early studies have also shown that patients with NERD may not respond as well to PPIs as patients with erosive disease . One study compared omeprazole 10 or 20 mg once daily with placebo in patients with heartburn , but without endoscopic signs of esophagitis [40]. Following 4 weeks of treatment, only 46 and 31 % of patients in the 20 and 10 mg groups, respectively, reported complete absence of heartburn, while 13 % in the placebo arm reported absence of heartburn. While superior to placebo, the rate of symptomatic relief was lower than reported in most erosive esophagitis trials. A second study of 209 patients comparing omeprazole 20 mg daily to placebo found similar results [41]. Following 4 weeks of treatment, only 43 % of patients were completely asymptomatic from heartburn and regurgitation , again a lower rate than most erosive esophagitis trials. Another study compared a 4-week trial of omeprazole 20 mg and 10 mg once daily in 277 patients with erosive esophagitis and 261 patients without erosive esophagitis [42]. Only 29 % of patients with nonerosive disease reported complete symptom relief on omeprazole 20 mg at 4 weeks, while 48 % of patients with erosive esophagitis reported relief.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






