Endoscopic score
Endoscopic findings
i0
No lesions
i1
≤5 aphthous lesions
i2
>5 aphthous lesions with normal mucosa between the lesions, or skip areas of larger lesions or lesions confined to the ileocolonic anastomosis (i.e., <1 cm in length)
i3
Diffuse aphthous ileitis with diffusely inflamed mucosa
i4
Diffuse inflammation with already larger ulcers, nodules, and/or narrowing
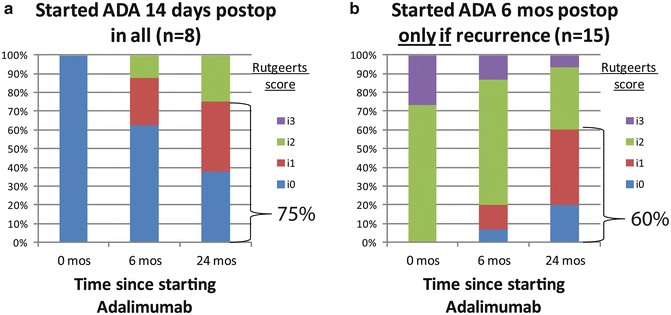
Fig. 13.1
(a) Endoscopic outcome of empiric versus (b) endoscopically guided postoperative prophylactic therapy with adalimumab (ADA), showing similar rates of endoscopic remission (Rutgeerts i0 or i1) 24 months after initiatingadalimumab [29]
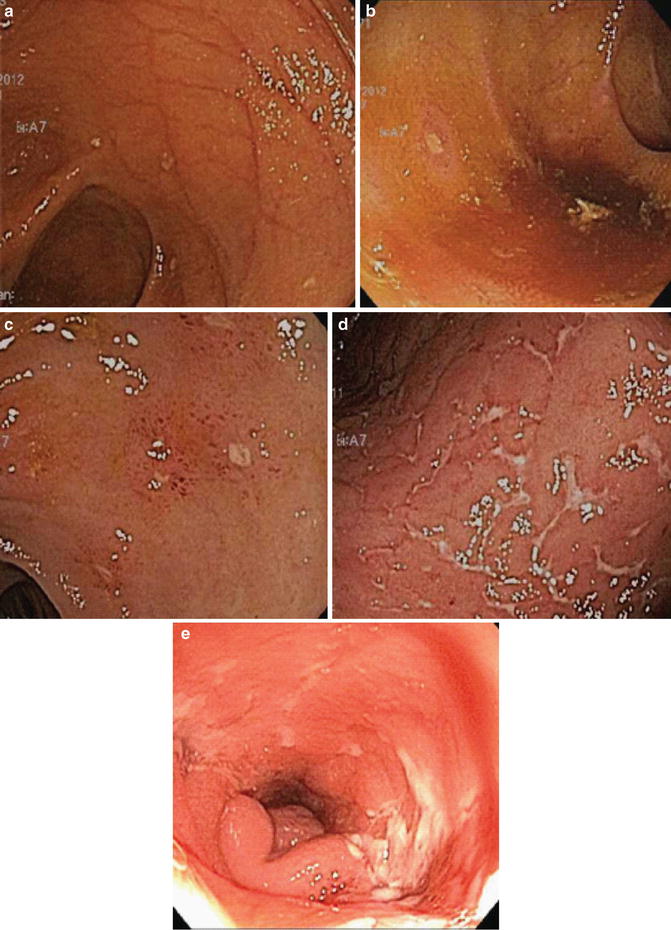
Fig. 13.2
Examples of Rutgeert’s scoring in postoperative recurrence of Crohn’s ileitis after ileocolectomy with primaryreanastomosis. (a) Score of i0, revealing normal ileal mucosa with no aphthous ulcers. (b) Score of i1, with rare (<5) aphthous ulcers on otherwise normal mucosa. (c) Score of i2, with 5 or more aphthae. (d) Score of i3, with diffuse aphthous ileitis and inflamed intervening mucosa. (e) Score of i4, with large, deep ulcers and luminal narrowing
Other common endoscopic findings after ileocolectomy include mucosal erythema and anastomotic ulcers. Although patchy erythema, sometimes with mucosal friability, was one of the earliest postoperative signs of inflammation to appear after surgery, Rutgeerts et al. deemed it too subjective and irreproducible to be included in a scoring system [4]. In contrast, anastomotic ulcers and stenosis appear to be a later postoperative change, as these changes are rare within the first year after surgery, even among patients with visible recurrence in the neoterminal ileum. Over time, anastomotic changes become much more common, and, at some point more than 3 years after surgery, as many as half of Crohn’s patients may develop a rigid, ulcerated stenosis of the anastomosis or neoterminal ileum, with serpiginous or longitudinal ulcers not uncommonly extending distally from the anastomosis into the colonic mucosa [4]. Thus, the endoscopic appearance of postoperative Crohn’s disease is not static, but instead progresses over time in the absence of prophylactic therapy [5].
In univariate analyses, the severity of early endoscopic recurrence (within 1 year of surgery, as reported by the Rutgeert’s score), predicts postoperative clinical recurrence better than symptoms, indication for surgery, size of colonic resection, disease duration, patient demographics, or whether or not patients had undergone prior resection [12]. If patients were stratified by preoperative disease severity, endoscopic score was found to be the only independent variable with significant predictive value. A score of i0-i1 predicts a risk of clinical recurrence and/or surgery of less than 10 % within the first 8 years after surgery [12], indicating no significant need for chronic prophylactic immunosuppressive or anti-TNF therapy. A score of i2 correlates with a 20–40 % recurrence rate at 5 years, for which the benefits of systemic prophylactic therapy may outweigh the risks and justify its cost. A score of i3 increases this 5-year risk to 50–70 %, while for i4 recurrence at 5 years is a 100 % certainty, with 70 % of recurrences occurring within the first year after surgery (Fig. 13.3) [12]. Thus, for the trials of prophylactic therapy discussed previously, a score of i2 was used as the threshold for endoscopic recurrence.
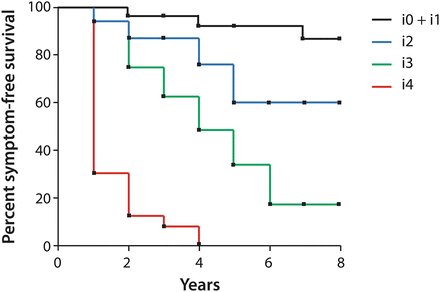

Fig. 13.3
Correlation between Rutgeerts score as assessed within a year of surgery, and subsequent clinical outcome. Adapted from [12]
For surgeries other than ileocolectomy, the natural history of postoperative Crohn’s disease is much less clear, but in general the rate of endoscopic recurrence appears to be lower and the rate of clinical recurrence higher than it is after ileocolonic anastomosis. In a retrospective, single-center series, endoscopic or radiographic recurrence, with a median follow-up of 12 months, was only seen in about 40 % of 38 Crohn’s patients who underwent surgery other than ileocolonic anastomosis [30]. Ileorectal anastomoses were included in this cohort, and behaved more like typical ileocolonic anastomoses, with five out of five patients demonstrating endoscopic recurrence at both the anastomotic site as well as the upstream neoterminal ileum. If these five patients were excluded from analysis, only one-third of patients showed endoscopic recurrence after ileostomy or colorectal, ileoileal, or more proximal small bowel anastomoses [30]. As with ileorectal anastomoses, all Crohn’s recurrences observed after anastomoses involving exclusively small bowel involved both the anastomosis and the adjacent small bowel. In contrast, recurrences after colocolonic anastomoses were limited to the anastomosis alone, with the adjacent colon upstream and downstream remaining healthy and uninflamed (Fig. 13.4). All of these patients with endoscopic recurrence developed clinical recurrence as well [30], so unlike ileocolonic anastomoses, other surgeries do not appear to produce subclinical endoscopic recurrence. Thus, whether endoscopic restaging in asymptomatic Crohn’s patients would have predictive value after surgery without an ileocolonic anastomosis is not clear. Instead, endoscopy after such surgery may be more useful to evaluate patients after symptoms have recurred, as a means of diagnosing the etiology of their symptoms and guiding therapy reactively instead of prophylactically.
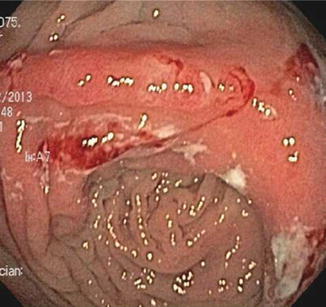

Fig. 13.4
Example of anastomotic ulceration as the sole evidence of recurrent Crohn’s disease in a symptomatic patient evaluated within a year of colocolonic anastomosis. Note that colonic mucosa proximal and distal to the anastomosis is normal and uninflamed
Thus, if endoscopy is to be used to evaluate for endoscopic recurrence and guide postoperative medical therapy in Crohn’s disease, it should ideally be performed within 6 months after surgery. Colonoscopy should be completed to the neoterminal ileum and the endoscopist should pay careful attention to the anastomosis and pre-anastomotic ileum. The pre-anastomotic ileum should be evaluated for evidence of recurrent disease and note should be made of the Rutgeerts score, with a score of i2 or greater indicating endoscopic recurrence. While histologic activity may be patchy and has not been shown to correlate with rates of clinical recurrence, histologic samples may provide supporting evidence of disease recurrence. In addition, the anastomosis should be evaluated for evidence of ulceration and stenosis, although these findings will be uncommon within the first year after surgery. If a severe and short anastomotic stenosis is present, endoscopic balloon dilation (described in Chap. 21) may be used for treatment and to allow evaluation of the pre-anastomotic ileum. It should be noted that ulcerations at the anastomosis alone (marginal ulcers) are not uncommon more than 1 year after surgery, but in the absence of pre-anastomotic ileitis do not constitute Crohn’s disease recurrence. While colonoscopy is currently the “gold standard” for identifying postoperative recurrence of Crohn’s disease following ileocolonic anastomosis, a number of less-invasive diagnostic techniques have been explored as an alternative. Fecal calprotectin correlates well with clinical recurrence, and better than serological testing, such as C reactive protein [31]. However, the correlation between endoscopic recurrence and fecal calprotectin has been variable [31–34]. Cross-sectional imaging, such as computed tomography (CT) and magnetic resonance (MR) enterography, are able to identify evidence of Crohn’s disease recurrence in regions not easily reached by conventional endoscopy, such as the mid small bowel [35]. However, these modalities primarily detect late postoperative changes, such as stricturing, bowel wall thickening or other anastomotic changes [36], and would be less sensitive than direct endoscopic visualization to identify early aphthous ulcerations predictive of subsequent clinical course. Small intestinal contrast ultrasound (SICUS) performed favorably alongside colonoscopy at 6, 12 or 24 months after ileocolonic anastomosis. Bowel wall thickening of >3.5 mm by SICUS, or the length such thickening extends into the neoterminal ileum, demonstrated excellent correlation with Rutgeerts scores of i2 or greater [37], and hence may have similar predictive power to colonoscopy in highly experienced centers.
Given the fact that Crohn’s disease recurrence is almost inevitably in the small bowel upstream of the ileocolonic anastomosis, wireless capsule endoscopy (WCE) has been proposed as a less invasive means to endoscopically visualize the neoterminal ileal mucosa than conventional colonoscopy. Like cross-sectional imaging, WCE has the added benefit of evaluating proximal small bowel beyond the reach of a colonoscope [38, 39]. However, because intestinal strictures increase the risk of capsule retention or impaction, WCE may need to be reserved for patients in whom fibrostenotic disease can first be excluded by cross-sectional imaging or a patency capsule. The latter is a radio-opaque capsule of the same dimensions as a WCE capsule, containing a small radio frequency identification (RFID) tag. If either abdominal plain films or the RFID tag demonstrate retention of the patency capsule in the small bowel 30 h after ingestion, it is presumed to have detected a stricture contraindicating WCE, but will subsequently dissolve, thus preventing obstruction and obviating the need for its removal. In a small, early series of postoperative Crohn’s patients with no radiographic or endoscopic evidence of stenosis, WCE appeared to be safe and well-tolerated, revealing mucosal recurrence in most cases [40].
Direct comparison of WCE and conventional colonoscopy 6 months after ileocolonic anastomosis demonstrated significant correlation when both employed Rutgeerts scoring, although WCE tended to report a lower score in a given patient, and thus had inferior sensitivity relative to ileocolonoscopy [39]. This study also revealed significant inter-observer variability in WCE scoring, highlighting the subjectivity of WCE results. A smaller study directly comparing WCE, colonoscopy, and SICUS 12 months after surgery demonstrated perfect correlation between WCE and conventional endoscopy, and a near-perfect correlation with SICUS, although this population demonstrated unusually aggressive recurrence, with endoscopic activity in 94 % of subjects, and anastomotic stricturing already present in 23 %, precluding WCE evaluation [38]. Furthermore, aphthous ulcers in the proximal small bowel, beyond the reach of a colonoscope, were identified in 76 % of patients. Thus, WCE may represent an attractive, less invasive mechanism for postoperative risk-stratification than conventional colonoscopy in Crohn’s disease.
Ulcerative Colitis
Although recent advances in medical therapies have created more options for the medical management of ulcerative colitis, 10–30 % of patients require colectomy in their lifetime [41–43]. The most common indications for colectomy in UC are medically refractory disease and colitis-associated cancer or dysplasia. Surgical options include total proctocolectomy with end-ileostomy or restorative proctocolectomy, most commonly with ileoanal pouch anastomosis (IPAA). While there can be surgical complications related to total proctocolectomy with end-ileostomy, surgery is curative and recurrence of inflammation postoperatively is generally felt to be reflective of unrecognized preoperative Crohn’s disease. Restorative proctocolectomy with IPAA involves formation of the ileum into a reservoir that is anastomosed to the anal transition zone. Post-IPAA inflammation is common and comes in several forms including cuffitis, pouchitis, and Crohn’s disease of the pouch. Unlike postoperative Crohn’s disease, there are no recommendations for routine endoscopy in the post-IPAA patient to evaluate for the recurrence of inflammation in the absence of symptoms. However, endoscopy is recommended in patients presenting with pouch dysfunction characterized by increased stool frequency, reduced form, rectal bleeding, urgency, cramping or incontinence. Endoscopic evaluation of the ileoanal pouch is critical to differentiate those entities that can cause postoperative inflammation and to exclude infectious or non-inflammatory causes of pouch dysfunction. Pouchoscopy can generally be accomplished without sedation, although the use of 2 % lidocaine may be helpful in alleviating discomfort in the anal area during the procedure. Preparation is achieved with a phosphate enema or half-dose of polyethylene glycol-based colonoscopy preparation. A narrower-caliber pediatric colonoscope or gastroscope is preferred as there is often mild stenosis at the anal-pouch anastomosis and these scopes have greater flexibility for retroflexion in the pouch. Please refer to Chap. 15 for details on post-IPAA anatomy. During pouchoscopy, the anal canal, ileal pouch-anal anastomosis, pouch and pre-pouch ileum are carefully assessed for evidence of inflammation. The exam should include a digital rectal examination to evaluate for anastomotic stricture and evidence of tenderness in the anal canal that could suggest abscess, fistula or ulceration in this area. In subjects with a stapled anastomosis, where a residual rectal cuff may be present, the length of columnar mucosa present between the anal transition zone and anastomosis should be noted. The anal canal, pouch and pre-pouch ileum should be assessed for endoscopic evidence of inflammation including granularity, friability, contact bleeding, ulceration and pseudopolyps. In addition any sinus tracts or fistulae should be identified. Biopsies should be taken of abnormal appearing mucosa during the exam. However, biopsy of the anastomoses and suture line ulcers should be avoided. These commonly exhibit histologic evidence of inflammation and foreign body granulomas, which may lead to misdiagnosis of pouchitis or Crohn’s disease of the pouch. Biopsies of the normal-appearing pouch and pre-pouch ileal mucosa can occasionally reveal histologic evidence of inflammation. Therefore, our practice is to take routine biopsies of these areas even when they appear endoscopically normal
Cuffitis
Cuffitis is the term used to describe inflammation of the residual columnar rectal mucosa left intact after IPAA with stapled anastomosis. During a hand-sewn anastomosis, a mucosectomy is typically performed of the distal anorectum and the pouch is sutured directly to the anal transition zone. A stapled anastomosis, alternatively, uses a stapling device to secure the pouch to the anorectal stump without mucosectomy. This often necessitates leaving a small “cuff” of residual intact rectal mucosa distal to the anastomosis that is at risk to become inflamed. While very uncommon, cuffitis can occur in patients who have undergone mucosectomy if islands of rectal mucosa have been left behind [44]. The frequency of cuffitis following stapled anastomosis is reported to occur in 7–15 % of patients [45, 46]. Cuffitis is probably best thought of as residual, rather than recurrent, inflammation and is thought to represent the same pathophysiologic process present in the preoperative colon of patients with ulcerative colitis.
The symptoms of cuffitis may include diarrhea, urgency, incontinence, rectal bleeding, and anorectal pain. Cuffitis is diagnosed by endoscopic findings of erythema, edema, increased granularity, decreased vascular pattern, friability and contact bleeding in the rectal cuff. Histologic evaluation reveals neutrophilic and chronic inflammatory infiltrates in the retained rectal mucosa (columnar epithelium between the anal transition zone and anastomosis). Cuffitis is more frequently symptomatic with a long segment of in situ rectum, but can occur with residual rectum of any length. Extraintestinal manifestations are more common in patients with cuffitis than other disorders of the pouch [47]. Cuffitis is treated similarly to proctitis, with first-line use of mesalamine and steroid suppositories [48]. However, recent data from a tertiary care center has suggested that up to 48 % of patients may be refractory to topical therapy. Many of these patients were later diagnosed with Crohn’s disease of the pouch or surgical complication such as fistula [49]. Thus, in patients with cuffitis who do not improve on topical therapies, further investigation for secondary causes of inflammation is worthwhile. Surgical revision of the ileal pouch-anastomosis has been reported to be successful in cases of refractory cuffitis with long segment of retained rectum [50, 51].
Pouchitis
De novo inflammation of the ileal reservoir, called pouchitis, is the most common cause of recurrent inflammation in post-IPAA patients. At least one episode of pouchitis is estimated to occur in up to half of patients with ulcerative colitis who undergo IPAA [52–57] and of these, 70 % develop pouchitis within the first year after ileostomy takedown [58]. The clinical symptoms associated with pouchitis overlap with other pouch inflammatory disorders and include increased stool frequency and liquidity, urgency, incontinence, anorectal pain, and/or abdominal cramping. Rectal bleeding and fevers are uncommon. Pouchitis has been classified according to several different clinical parameters. The clinical course of pouchitis can be acute, relapsing or chronic. Forty percent of patients will have only a single episode of acute pouchitis [59], while 5–19 % of patients will develop chronic pouchitis defined as lasting more than 4 weeks [60–62]. Pouchitis can be additionally classified according to antibiotic responsiveness (antibiotic responsive, antibiotic dependent, or antibiotic refractory).
Dysbiosis, or alteration in the composition or quantity of bacteria in the ileoanal pouch, has been strongly implicated in the etiology of pouchitis. This is evidenced by the fact that pouchitis rarely occurs in the absence of the fecal stream and that both probiotics [63, 64] and antibiotics [65, 66] are effective for the treatment of pouchitis. Decreased bacterial diversity and changes in the bacterial composition have been reported in subjects with pouchitis after IPAA, although no specific species or phylotypes appear to be causative [67, 68]. Host factors, including genetics, also appear to play an important role as pouchitis rarely occurs in patients undergoing IPAA for familial adenomatous polyposis [69–71].
Clinical risk factors predictive of development of chronic pouchitis include young age at colectomy, never smoking status, NSAID use, extensive disease and backwash ileitis [58, 72, 73]. In addition, concurrent primary sclerosing cholangitis (PSC) as well as the presence of other immune mediated disorders has [74] been found to be a significant risk factor in the development of pouchitis [58, 75, 76]. Genetic polymorphisms have been associated with risk of pouchitis including those in TLR9, CD14, TLR1 and NOD2 [72, 77, 78]. A recent meta-analysis of eight studies, revealed an association (odds ratio of 1.76) of anti-neutrophil cytoplasmic antibody (ANCA)-positivity with chronic, but not acute, pouchitis. No similar association was seen with anti-Saccharomyces cervisiae antibody [79]. Seropositivity for anti-CBir1 [80] and anti-outer membrane porin (OmpC) [72] have additionally been associated with risk of pouchitis development.
The diagnosis of pouchitis is based on the combination of clinical, endoscopic and histologic findings. Characteristic endoscopic findings compatible with pouchitis are diffuse or patchy erythema, friability, edema, hemorrhage, granularity, exudates and ulceration (Fig. 13.5). Linear ulcers can be found at the anastomosis and should not be interpreted as evidence of pouchitis. Endoscopy allows for determination of the severity and extent of inflammation, including evaluation of the rectal cuff and pre-pouch ileum. It should be noted that pre-pouch backwash ileitis has been associated with diffuse pouchitis. Therefore, the presence of ileitis does not necessarily portend a diagnosis of Crohn’s disease of the pouch. However, lack of significant pouchitis, deep ulceration or extent of ileitis beyond the distal pre-pouch ileum should raise the suspicion for Crohn’s disease of the pouch. In addition, cuffitis may occur in patients with pouchitis [45] and can represent a secondary phenomenon that responds to primary treatment of pouchitis in some cases.
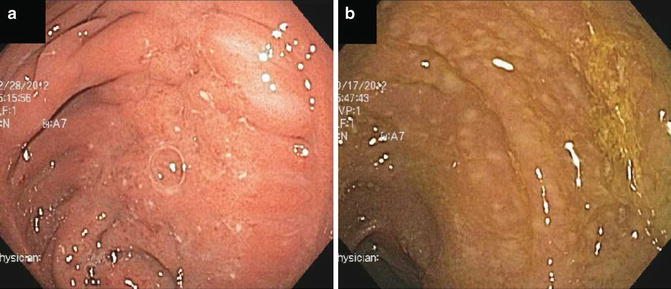

Fig. 13.5
Examples of pouchitis. (a) Idiopathic pouchitis. (b) Pouchitis secondary to Clostridium difficile infection
Endoscopic appearance does not necessarily correlate well with histologic severity, likely related to issues of sampling error [81]. Histologic findings associated with pouchitis are non-specific and include acute inflammation with neutrophilic infiltrates, crypt abscesses and ulceration as well as chronic inflammatory infiltrates. Inflammation is often associated with villous atrophy and crypt hyperplasia [82, 83]. Although histologic findings are not specific for pouchitis, evaluation can alert the endoscopist to alternative causes of pouch inflammation if granulomas, viral inclusion bodies (suggestive of cytomegalovirus) or evidence of ischemic changes are identified. As infections, NSAID use and ischemia can mimic the endoscopic and histologic findings of idiopathic pouchitis; these entities should be considered as causes of secondary pouchitis, particularly in those patients with relapsing and antibiotic-refractory disease.
First-line therapy for the treatment of acute pouchitis is accomplished with a 2 week course of ciprofloxacin or metronidazole. Both metronidazole (15–20 mg/kg/day) [65] or ciprofloxacin (1 g/day) have been shown in randomized controlled trials to be effective for the treatment of acute pouchitis, with ciprofloxacin demonstrating superior efficacy and fewer side effects in a comparative efficacy study [66]. The probiotic VSL#3 has also demonstrated efficacy in randomized controlled trials for the treatment of symptoms from mild pouchitis [63]. Budesonide enemas have also been shown to be equivalent to metronidazole in a randomized comparative efficacy study and were better tolerated [84]. In randomized controlled trials of patients with antibiotic-dependent pouchitis and relapsing pouchitis, VSL #3 was also shown to be effective in maintaining antibiotic-induced remission in 85 % of patients [64, 85]. However, subsequent open-label studies have demonstrated less impressive results [86]. Open-label studies of antibiotics including rifaxamin have also suggested a possible role in maintenance therapy [87, 88].
Chronic antibiotic-refractory pouchitis is difficult to treat and unfortunately remains a major cause of ileoanal pouch failure. In the patient who has failed antibiotic therapy for pouchitis, causes of secondary pouchitis should be sought including evaluation for NSAID use, ischemia, structural disease, infections (particularly Clostridium difficile and cytomegalovirus) and Crohn’s disease of the pouch. Some groups have suggested stool cultures may be helpful in documenting antibiotic resistance and directing antibiotic therapy in this group of patients [89]. Combination antibiotic therapy with Cipro and Rifaximin [90, 91], metronidazole [92] or tinidazole [93] for 1 month had been used to achieve remission in open-label studies. However, maintaining remission in this group of patients remains a major challenge. Small case series have described the successful use of steroids (beclomethasone [94], budesonide [95]), immunosuppressive agents, topical tacrolimus [96] and anti-TNF [97–100] agents in this population. However, the role of anti-TNF agents remains controversial as the patients treated in these studies were heterogeneous and included patients with significant pre-pouch ileitis and fistulizing pouchitis, raising a question of whether those who responded to therapy had Crohn’s disease of the pouch rather than idiopathic pouchitis.
Several small randomized controlled studies have suggested a role for probiotics in the primary prevention of pouchitis [101, 102]. However, larger studies including high- and low-risk populations will need to be evaluated to determine whether primary prophylaxis of pouchitis provides long-term benefit and is cost effective.
Crohn’s Disease of the Pouch
Crohn’s disease of the pouch, alternatively called Crohn’s-like disease of the pouch, is another leading cause of post-IPAA inflammatory disease. Crohn’s disease of the pouch is characterized by fistulizing or stricturing disease of the pouch and/or extensive inflammation of the afferent limb. Crohn’s disease of the pouch is reported to occur in 3–13 % of patients undergoing IPAA, with higher rates in patients with indeterminate colitis [103–108]. Crohn’s disease of the pouch can be diagnosed early (within weeks) or late (years) after IPAA. Clinical symptoms may be similar to other pouch inflammatory diseases and include increased stool frequency, liquidity, urgency, incontinence, abdominal pain, rectal pain. In addition, patients may have obstruction associated with strictures, draining fistulae or malabsorptive phenomena in the setting of extensive small bowel involvement. Based on the pattern of involvement, Crohn’s of the pouch can be classified into inflammatory, fibrostenotic and fistulizing phenotypes.
There is significant debate as to whether Crohn’s disease of the pouch after IPAA for ulcerative colitis reflects a missed preoperative diagnosis of Crohn’s disease or may occur de novo in susceptible individuals. Patients with indeterminate colitis and those with a family history of Crohn’s disease have increased risk of Crohn’s disease of the pouch, suggesting the former possibility in some patients. Yet many patients who develop this entity have no preoperative clinical features of Crohn’s disease or evidence of Crohn’s-like pathology on their colectomy specimens. Thus, it is possible that the post-surgical anatomy of IPAA creates a permissive environment for development of de novo Crohn’s disease in susceptible patients. In addition to family history of Crohn’s disease and preoperative indeterminate colitis, risk factors for Crohn’s disease of the pouch include young age at time of IPAA and active smoking [73]. Seropositivity for ASCA and CBIr1 also increases the risk of Crohn’s disease of the pouch [109, 110]. Interestingly, the presence of PSC appears to protect against the development of Crohn’s disease of the pouch [111].
Crohn’s disease of the pouch can affect the cuff, pouch and/or pre-pouch ileum, but is most commonly active at the anastomoses and pre-pouch ileum. In addition, inflammation can be present in the proximal small bowel and upper GI tract. Endoscopic findings are characterized by edema, granularity, friability, contact bleeding, pseudopolyps, exudates and deep ulcerations (Fig. 13.6). Features that distinguish Crohn’s disease from other inflammatory conditions of the pouch include strictures (particularly non-anastomotic strictures), fistulas and extensive ileitis [112]. Ileitis that is more than 10 cm proximal to the pouch-ileal anastomosis or bears discrete ulcerations or is non-responsive to antibiotic therapy should raise the suspicion of Crohn’s disease. Upper GI involvement on esophagogastroduodenoscopy or mid-small bowel disease identified on wireless capsule endoscopy are also suggestive of Crohn’s disease. Radiographic evaluation of the pouch with MRI or CT can be of additional benefit in identifying stricturing or penetrating complications of Crohn’s disease.
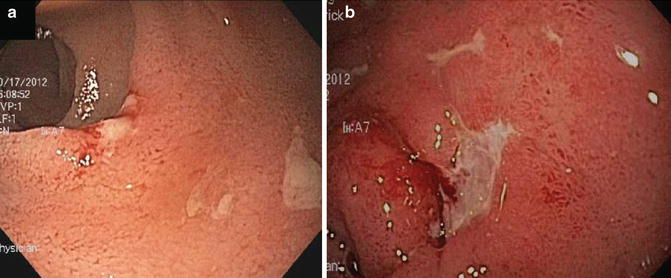

Fig. 13.6
Examples of Crohn’s disease of the pouch. (a) Discrete ulcerations in pre-pouch ileitis. (b) Crohn’s disease of the pouch associated with deep ulcerations in the pouch body
Histologic evaluation can be diagnostic of Crohn’s disease of the pouch when granulomas are identified. However, only 10–12 % of patients diagnosed with Crohn’s disease of the pouch will have granulomas [113]. Anastomotic biopsies should be avoided during pouchoscopy, as foreign-body granulomas can be present in these areas and may not reflect a diagnosis of Crohn’s disease of the pouch. Other histologic features have been proposed to distinguish pouchitis and Crohn’s disease of the pouch including pyloric gland metaplasia (PGM). PGM is more common in Crohn’s disease than pouchitis, but the sensitivity and specificity of this finding (77 and 78 % respectively) are not high enough to warrant its use as a diagnostic tool [114].
The diagnosis of Crohn’s disease of the pouch can be difficult to make as it shares endoscopic and histologic features with other inflammatory disorders of the pouch. The features that traditionally help distinguish Crohn’s disease from ulcerative colitis can be less reliable post-IPAA where surgical manipulation can result in both stricturing and fistulizing complications. In addition, patients with pouchitis may have patchy distribution and transmural inflammation, making these less reliable features of Crohn’s disease of the pouch. Furthermore, pouchitis and backwash ileitis can mimic Crohn’s disease of the pouch. However, ileitis that is long-segment (>10 cm), or has discrete ulcerations or a different mucosal pattern than inflammation of the pouch or occurs in the absence of pouchitis heightens the suspicion of Crohn’s disease. Response to antibiotics can also help distinguish backwash ileitis from Crohn’s disease of the pouch. Crohn’s disease of the pouch can also affect the rectal cuff and should be considered in cuffitis that is not responsive to topical therapies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








