Fig. 16.1
MRI images in TilePro (bottom) and operative field (top)
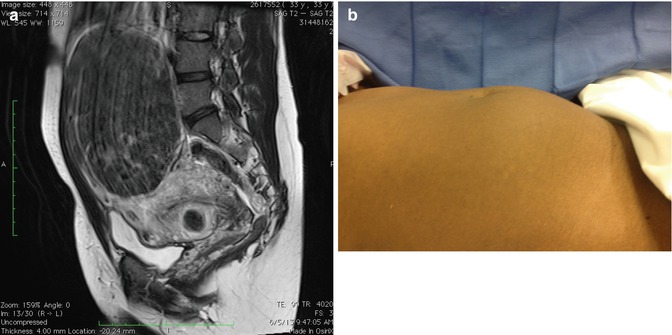
Fig. 16.2
(a) A 20–22-week size uterus with large fundal myoma on sagittal view MRI. (b) View of abdomen in same patient before surgery
16.4 Technique
16.4.1 Preparing the Operative Field
Robotic surgery starts like any other laparoscopic procedure, with the placement of a uterine manipulator and laparoscopic ports. The location of the trocar incisions depends on the size and number of the myomas and the number of robotic instruments used to carry out the procedure. The number of ports can vary. One can use five ports if all robotic instruments are used, including the third instrument arm and an assist port; four if only two robotic instruments are used with one accessory port; and three (without an assist port) and even two ports if reduced port robotic myomectomy techniques are employed (Fig. 16.3a–d). Operator experience and the size of the pathologic tumor being addressed basically determine which modality is leveraged. In addition, the patient’s concerns regarding cosmesis may influence the decision in terms of how many ports are placed and if the camera port or the largest port is placed in the umbilicus.
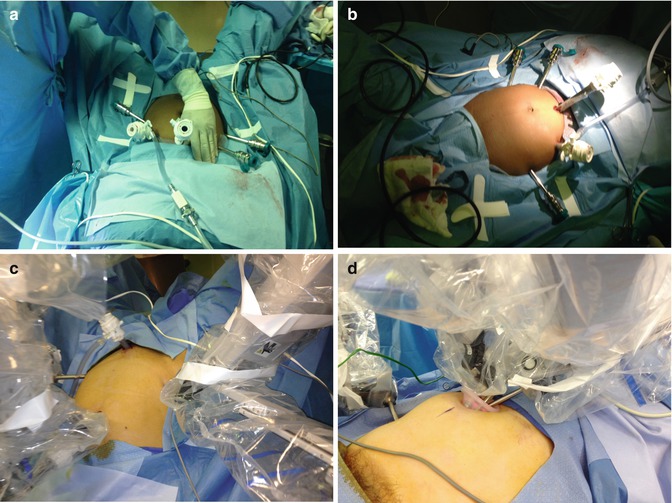


Fig. 16.3
(a) Multiport robotic surgery—instrument ports spaced one hands-breadth apart. (b) Multiport robotic surgery—two instruments on the right side. (c) Reduced port robotic surgery with supra pubic assistant trocar. (d) Single umbilical incision plus 5 mm robotic instrument right lower quadrant. (e) Supra pubic assistant trocar
16.4.2 Step-by-Step
Prior to starting the procedure, attention must be paid to ensuring some modicum of hemostasis during the operation for a uterus that is still well vascularized. A number of techniques have been described that preemptively reduce pulse pressure to the area surrounding the myoma or myomas to be removed. These include (1) constricting the blood flow from the uterine arteries via a tourniquet by opening up the leaves of the broad ligament bilaterally and applying either sutures placed endoscopically or a Penrose drain. (2) Another technique involves the injection of a dilute solution of vasopressin into the area surrounding the intended hysterotomy incision, allowing the effects of the fluid to both create a plane for the enucleation of the fibroid and effecting vasoconstriction of the vessels supplying the myoma(s). In some countries (outside of the U.S.), however, the use of vasopressin is not allowed because of reports of serious and potentially fatal side effects of the drug. Therefore it is important to avoid direct intravascular injection of the mixture.
This can be accomplished by (1) introducing a catheter with a needle through one of the trocars and directing the medication to the intended area, or (2) a method preferred by the author that involves direct transcutaneous transabdominal instillation of the drug using a 22-gauge, 5-in (or 7-in) spinal needle and robotic instruments to guide the process (Fig. 16.4). The surgeon or assistant should first aspirate; then once it is evident that the intravascular space is not breached, the vasopressin may be injected in small aliquots. A typical mixture would be 20 U of vasopressin placed in 60 mL of normal saline. No more than 60 mL of the solution should be administered. If more is required, an interval exceeding the half-life of the drug should be used as a guide in terms of timing.
There are four basic steps to accomplishing a robotic myomectomy:
1.
Hysterotomy incision(s)
2.
Enucleation of the myoma(s)
3.
Repair of the defect(s)
4.
Extraction of the myoma(s).
After securing a method of prophylactic hemostasis, the hysterotomy incision may be created. The decision of creating a transverse versus a vertical versus an elliptical incision depends on the location and type of the fibroid. For broad-based exophytic myomas it may be preferable to perform an elliptical incision at a level from the base to allow for room for adequate myometrium for the repair of the defect. Transverse hysterotomy incisions (Fig. 16.5), especially on anterior myomas, may be preferable given the historical data on the strength of the closure from the experience from low transverse cesarean sections and the potential for uterine rupture rates versus vertical hysterotomy incisions during subsequent pregnancies. It must be noted, however, that there is an association between anterior hysterotomy incisions, large myomas, and the incidence of preterm labor for patients who become pregnant following robotic myomectomy [10].
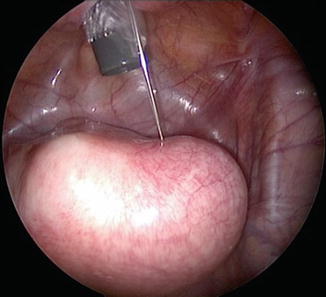
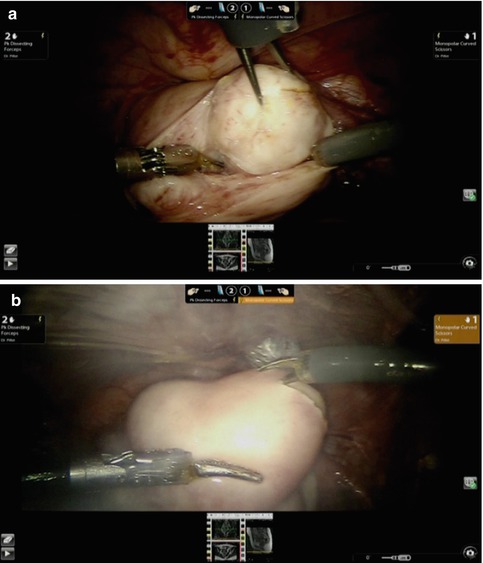

Fig. 16.4
Injecting vasopressin prior to hysterotomy incision

Fig. 16.5
(a) Enucleation using mechanical forces. (b) A transverse hysterotomy incision with “pure cut” energy setting
16.4.2.1 Hysterotomy Incision
The instrument of choice for the hysterotomy incision is usually monopolar curved shears. Other monopolar robotic instruments may be used, but whatever tool is chosen should be used efficiently, leveraging the principles of electrosurgery such as power density. This means that the energy delivered by the tip of the instrument is inversely proportional to its diameter, and a relatively low power setting in terms of wattage can be used to effectively cut through the serosa with the least amount of lateral thermal spread. Obviously the use of a CO2 laser and/or a harmonic scalpel can result in less lateral thermal spread, but the difference may not be clinically significant. In addition the harmonic scalpel is not a wristed instrument and poses some challenges when used with other robotic instruments. The recommended energy settings are dependent on the generator’s being used and the available data from the manufacturer.
Once the hysterotomy incision is created, it must be made at a uniform depth to the level of the pseudocapsule of the myoma and not protrude into the myoma itself. This may be accomplished by using a curved dissecting bipolar device such as the PK Dissector (Intuitive Surgical, Inc.; Sunnyvale, CA). The fenestrated bipolar device does not lend itself to fine dissection for robotic myomectomy. A list of recommended instruments is shown in Table 16.1.
Table 16.1




List of instruments used for robotic myomectomy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








