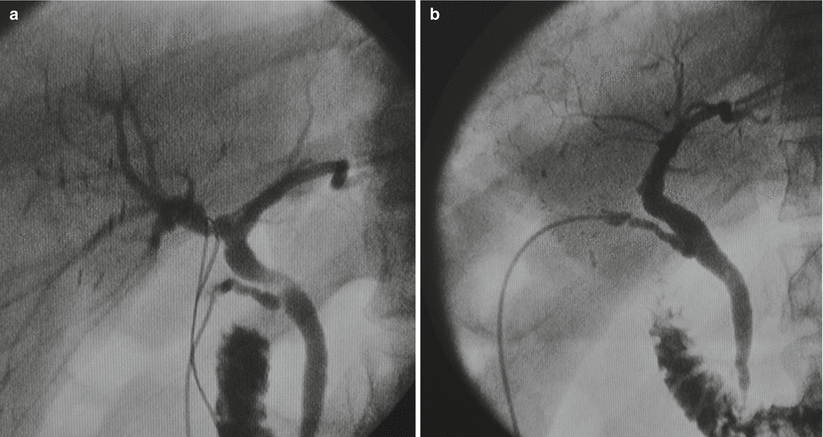
Fig. 28.1
Classification and incidence of the hepatic artery anatomy: type1, 71 %; type2a, 6 %; type2b, 6 %; type3a, 3 %; type3b, 10 %; type4a, 0 %; type4b, 2 %; type5, 1 %. AO aorta, CA celiac axis, CHA common hepatic artery, GDA gastroduodenal artery, LGA left gastric artery, LHA left hepatic artery, PHA proper hepatic artery, RHA right hepatic artery, SA splenic artery, SMA superior mesenteric artery
The dominant artery of segment IV (quadrate lobe) is also the vital screening content for LDLT using right lobe. It may arise from left hepatic artery, right hepatic artery, proper hepatic artery, even at the same time from left and right hepatic artery. If the artery of segment IV is from right hepatic artery, the distance between it and right hepatic artery origin should be determined, and the transection plane should avoid affecting its blood supply for segment IV.
28.5.2 Portal Venous Anatomy
Anomalous branching pattern of the portal vein (PV) at the hepatic hilum is less frequent than those of the hepatic arteries, hepatic veins, and biliary ducts [41]. And their incidence has been reported about 30 % in previous publications [42, 43]. The normal PV anatomy is defined as a division of the main portal vein into two branches: the left (supplying segments II, III, and IV) and right portal veins. The right portal vein divides secondarily into two branches: the right anterior portal vein feeding segments V and VIII and the right posterior vein feeding segments VI and VII. Any deviation from this anatomy is considered an anatomical variant. The anatomical variations of the portal vein are shown in Fig. 28.2 according to Chen et al. [44] and Covey et al. [45] classifications. Presurgical awareness of portal vein anatomy is utmost important for operation strategy. For example, RP form main and trifurcation, the two portal veins of right graft should be reconstructed during transplantation.
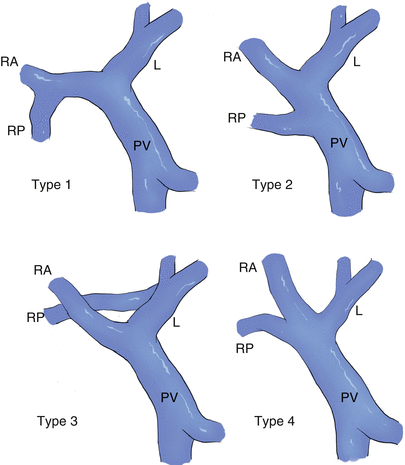

Fig. 28.2
Schematic guide for assignment of categories of conventional and variant portal venous anatomies: type1, 81 %; type2, 11 %; type3, 2 %; type4, 4 %; others, 2 %. RA right anterior branch, RP right posterior branch, L left branch, PV portal vein
28.5.3 Biliary Anatomy
Variations in biliary anatomy are not uncommon, with the classical branching pattern present only in about 60 % of the normal population; right hepatic duct drains from the right anterior or posterior sectoral duct [46]. The anomalies of right hepatic duct result in multiple graft bile duct openings and require more complicated biliary anastomosis in the recipient. Information regarding their biliary anatomy can guide appropriate surgical strategies or help in the selection of the optimal donor when multiple donor candidates are available. The anatomical variations of the biliary anatomy were described according to Choi et al. [47] and Varotti (Fig. 28.3) classifications.
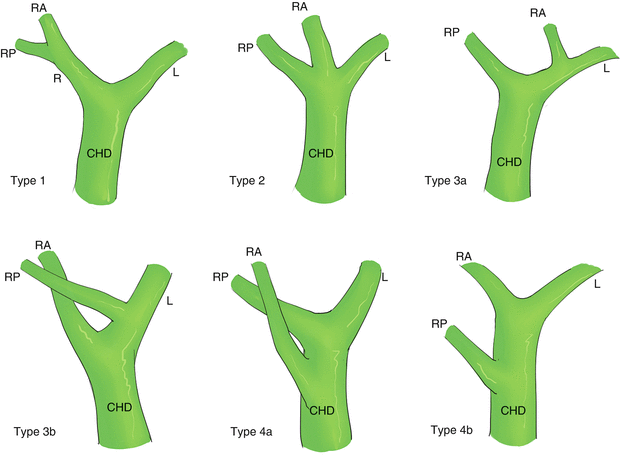

Fig. 28.3
Classification and incidence of the biliary tree anatomy: type1, 56 %; type2, 14 %; type3a, 5 %; type3b, 16 %; type4a, 3 %; type4b, 7 %. CHD common hepatic duct, L left hepatic duct, R right hepatic duct, RA right anterior hepatic duct, RP right posterior hepatic duct
28.5.4 Hepatic Vein Anatomy
Generally, the middle and left hepatic veins converging a common trunk, which open into the inferior vena cava, account for majority. The middle and right hepatic veins sharing the common trunk are not fit for right-lobe LDLT. Based on the presence or absence of significant segment V and VIII accessory hepatic veins and one or more accessory short hepatic veins, Varotti proposed four anatomic patterns [38] (Fig. 28.4). Inferior right hepatic veins and the tributaries of middle hepatic vein with diameter >5 mm should be kept for reconstruction during transplantation in case of severe congestion.
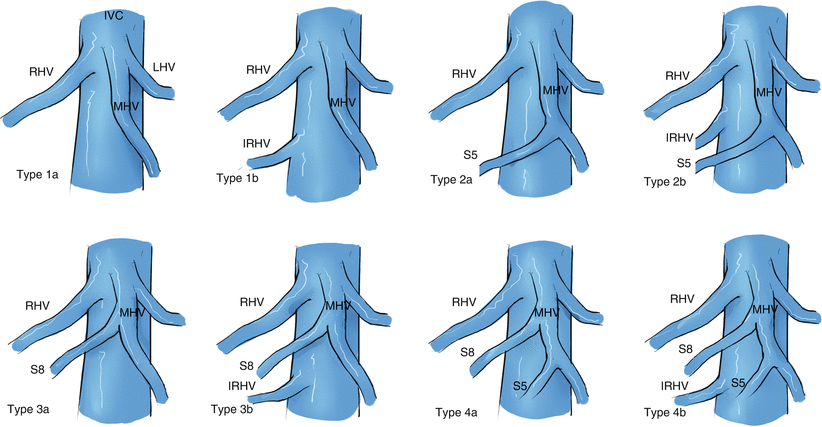

Fig. 28.4
Classification and incidence of the right liver hepatic venous anatomy. Type1: Absence of S5 and S8; absence of IRHV (type1a, 14 %), or presence of IRHV (type1b, 8 %). Type2: Presence of S5; absence of IRHV (type2a, 20 %), or presence of IRHV (type2b, 9 %). Type3: Presence of S8; absence of IRHV (type3a, 17 %), or presence of IRHV (type3b, 19 %). Type4: Presence of both S5 and S8; absence of IRHV (type4a, 11 %), or presence of IRHV (type4b, 3 %). IRHV inferior right hepatic vein, IVC inferior vena cava, LHV left hepatic vein, MHV middle hepatic vein, RHV right hepatic vein
28.6 Preoperative Preparation
Under the guidance of doctors, the donors are on liquid food the day before the planned transplantation and fast after midnight. The bowel preparation is also undergone the night before the planned transplantation. All these should keep the donor at his/her best without any health problems.
At the operative day, the transplant surgeon and the anesthesiologist review the donor data for the last time just before the operation. And the donors should receive an epidural patient-controlled analgesia for postoperative pain management.
To prevent deep venous thrombosis, the compressing equipment around lower extremities is applied immediately before general anesthesia with endotracheal intubation. After general anesthesia, urethral catheterization with Foley catheter and nasogastric tube drainage are performed. Antibiotic prophylaxis, the central venous pressure, and arterial blood gas analysis are usually necessary.
Preoperative autologous blood donation or intraoperative salvaged autotransfusion sometimes would be considered for special situations.
28.7 Operation Procedure
28.7.1 The Key Points of Graft Harvest
1.
A cavitron ultrasonic surgical aspirator (CUSA) is recommended for parenchymal dissection, particularly along the hepatic veins and major vessels. When closing with hepatic veins, the power of CUSA should be reduced, and division is carried with more caution. Sometimes intraoperative ultrasound evaluation of the vascular structures is needed for reconfirming liver transection plane and the anatomy of hepatic veins and inferior vena cava. CUSA with the higher power or rough action would easily cause rupture of the hepatic vein and massive hemorrhage.
2.
In principle, during the graft harvest, parenchymal transection is performed without the Pringle maneuver to reduce liver ischemia-reperfusion injury.
3.
The clamping site of atraumatic forceps on the vessels is very important. It cannot be too close to the main trunk of the portal vein when clamping its branches, in order to avoid portal vein stenosis and subsequent portal hypertension of the donor. Similarly, for hepatic veins, the forceps cannot be placed too close to the common trunk of the left hepatic vein and middle hepatic vein in case Budd-Chiari Syndrome occurs.
4.
Large inferior right hepatic veins are preserved and reconstructed in implantation; otherwise, adequate drainage of graft would be affected.
28.7.2 Laparotomy
A J-shaped right subcostal incision is firstly used for exploration. If liver texture is normal, the incision is expanded to a bilateral subcostal incision with the right longer than the left. Whether an upper midline extension (Mercedes incision) is necessary depends on donor’s costal arch angle, body type, and operative fields. Generally, Mercedes incision provides excellent access to liver but may delay or prevent healing at the junction of the vertical and horizontal incision then prolongs hospitalization. Therefore, we advocate finishing donor’s surgery through the bilateral subcostal incision if possible.
After entering the peritoneum, pathological condition and anatomical variations of the liver and surrounding tissues need to be carefully explored again (Fig. 28.5). Special attentions should be paid to anomalous bile ducts and hepatic arteries, abnormal enlargement of the lymph nodes and mass, varicose veins, left- and right-lobe size, surrounding ligaments, and liver texture and color. When necessary, intraoperative biopsy is required to determine whether the donor’s liver suits donation. Intraoperative ultrasound examination is performed to identify the major vascular structure of the liver. The anatomical variants of left and middle hepatic vein are common and usually present as a common trunk of the two veins before entering the inferior vena cava. The dissection begins until finishing these evaluations.

Fig. 28.5
Exploration after laparotomy
28.7.3 Cholecystectomy and Biliary Duct Assessment
After careful separation of the cystic artery and the cystic duct, the gallbladder is retrogradely removed. During this procedure, the surgeon should preliminarily understand the anatomy of extrahepatic bile ducts, including the distribution of the common bile duct and the left and right hepatic duct, and their bifurcations.
Cholangiography through the cystic duct stump for evaluation of the biliary tree is performed after cholecystectomy. We usually use the epidural catheter and put it into the common bile duct through cystic duct. However, due to a helical structure of the duct, the catheter is difficult to intubate and may cause the common bile duct injury in the donor. Therefore, we usually preserve Hartmann’s pouch for binding the catheter at the entrance of cystic duct with silk suture (Fig. 28.6). The routine intra-operative cholangiography is helpful to clarify the division line of the hepatic duct, reducing the biliary complication rate [48].
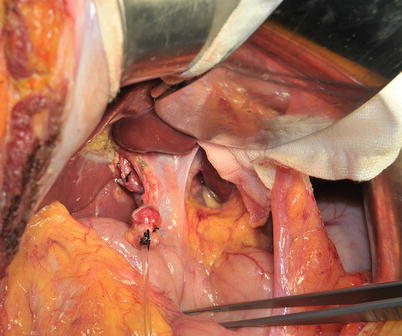

Fig. 28.6
Preserved Hartmann’s pouch for putting the catheter into cystic duct
The cholangiography using C-arm X-ray system is performed to delineate the biliary tree and second-order biliary duct branches (Fig. 28.7) as well as the variants.
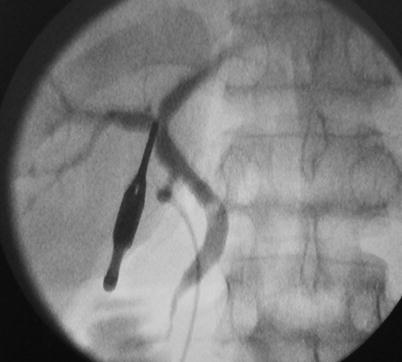

Fig. 28.7
Cholangiography before the separation of liver
28.7.4 Separation of Right Hepatic Artery
Anatomic variations of hepatic artery are the most common among variations of hilar structures. In the presence of any variations, the principles of graft surgery conclude:
1.
The arteries of the right-lobe graft must be preserved with appropriate length and size.
2.
No harm to the arteries and blood supply of the donor’s remnant liver.
3.
Variant hepatic arteries must be preserved as well as possible, since each hepatic artery dominates a specific area and their terminal branches doesn’t communicate with each other.
The right hepatic artery generally is located on the right side of the common bile duct. Originating from the proper hepatic artery, the right hepatic artery goes into the right liver through the backside of the common bile duct. Generally, it is lower than the bifurcation of the common hepatic duct and the portal vein. The classic anatomy is reported in about 55–75 % of the population. Separation is performed along the right hepatic artery until reaching the right lobe. And then label the right hepatic artery with a red rubber band (Fig. 28.8). Caution that directly clamping the artery and using electrocautery near the arteries should be avoided, in case of arterial injury.
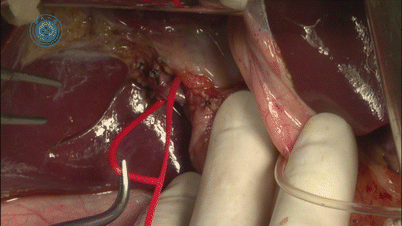

Fig. 28.8
Separation of right hepatic artery
During the procurement of right-lobe graft, the surgeon should identify and preserve any artery branches supplying blood to the segment IV. The artery of segment IV frequently arises from the left hepatic artery. But a few cases present the so-called middle hepatic artery from proper hepatic artery to supply segment IV and V, the middle hepatic artery should be retain for the donor. Beyond this, the artery of segment IV of some cases branches from the right hepatic artery; this branch sometimes supplies blood to the segments II and III. Therefore, the injury to this artery during donation would cause partial left liver ischemia, even leading to biliary leakage and liver atrophy. In this situations, the right hepatic artery should be separated enough long, and dissection of right artery should be after the origin of this branch. However, excessive dissection of biliary duct system and proximal right hepatic artery should be avoided to prevent biliary duct ischemia.
28.7.5 Separation of Right Portal Vein
The separation continues to the medial side of the hepatoduodenal ligament. By stripping a little connective tissue, the portal vein trunk and the right portal vein are present with the bluish purple side wall. Carefully ligate the superficial lymphatic ducts to prevent postoperative chylous leakage.
After confirming the right branch of the portal vein, surgeons use a blunt rectangular forceps to separate it from the bifurcation and confirm its extension direction. And then label the right branch of the portal vein with a purple rubber band (Fig. 28.9). Generally, right portal vein has relative long extrahepatic segment and is easy to be dissected free. But the surgeons should preserve adequate long stump for closure at the donor side to prevent stenosis in the main trunk of portal vein after the suture.
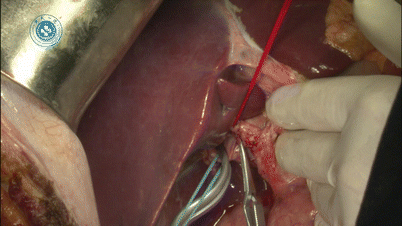

Fig. 28.9
Separation of right portal vein
Sometimes, in order to acquire enough length of free right portal vein, the dissection could be extended into the liver parenchyma during the operation, cutting off the veins originating from the right portal vein and supplying blood to the caudate lobe. Transecting small branches will not lead to death of the caudate lobe or biliary leakage.
Portal vein anomaly generally doesn’t require any modification during liver transection. The plasty of portal vein is performed using cryopreserved iliac and/or saphenous vein grafts in back table procedure, which is relative easy to dissolve.
The portal blood of the segment IV may come from the branch of the right portal vein. Although these abnormal branches to segment IV are rare, it is still important to clarify the branches and direction of right portal vein and be aware of such branches.
28.7.6 Right Lobe Separation and Parenchymal Transection
The dissection of liver ligament begins after the separation of right hepatic artery and portal vein.
Dissect the falciform ligament and the right triangle ligament, turn the donor liver to the left, and expose the posthepatic inferior vena cava. Separate the right adrenal gland from the liver, and transect and ligate the short hepatic veins. Separate the right hepatic vein and label it with a blue rubber band. During the process, surgeons may see some posthepatic veins and short hepatic veins draining segments VI and VII. The veins >5 mm in diameter need to be preserved. The approach is not to cut them until the transection of major vessels. This may cause some degree impact on the exposure of posthepatic structures, but most cases could be finished successfully. It is also feasible to transect the veins after seizing with atraumatic clamps. That depends on surgeon’s demands and preferences.
Until now, the right hepatic artery and portal vein of the first porta hepatis and the right hepatic vein of the second porta hepatis and the third porta hepatis have been successfully separated. After blocking right portal vein and right hepatic artery with atraumatic clamps, Cantlie’s line forms between left and right lobes. Mark the line by electrocautery and transect the liver (Fig. 28.10). The most common method for transection is CUSA (Fig. 28.11). The vessels are clipped or ligated and subsequently cut. The tributaries of middle hepatic vein >5 mm should be retained for reconstruction during transplantation in case of severe congestion (Fig. 28.12).

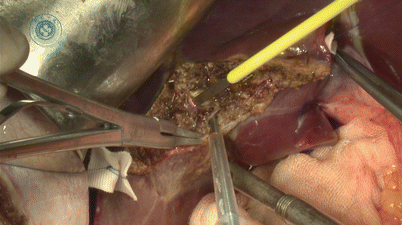
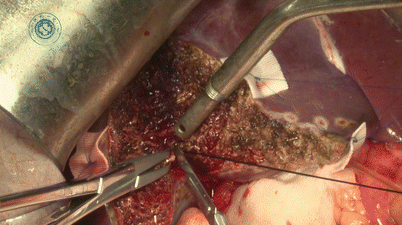

Fig. 28.10
Mark the transection line by electrocautery

Fig. 28.11
Liver parenchyma transection by CUSA

Fig. 28.12
The tributaries of middle hepatic vein >5 mm are preserved
28.7.7 Time Point for Bile Duct Transection
In the process of LDLT, transection of donor bile duct basically suggests that the donation cannot be aborted. This is the so-called point of no return.
Usually, donors’ operation begins ahead of recipients’. However, if the recipients died during the operation, or a variety of situations happen, such as severe adhesions, bleeding, and other conditions, the operation cannot proceed or eventually abort. Once these unexpected situations happen and the donor liver has already been cut, it will become an orphan donor. That means the graft will be taken by no recipient or someone else, resulting in the difficult-solved ethical issue. Therefore, it is necessary to determine an appropriate time point after which the donor’s liver must be transected. Currently, transection of the bile duct is believed to be such a time point.
The order of living-donor graft harvest is transection of most part of liver parenchyma, cholangiography and transection of the bile duct, transection of hepatic artery and portal vein, and transection of hepatic vein in the First Affiliated Hospital of Zhejiang University. Cholangiography is performed before and after dissection of right biliary duct (Fig. 28.13). Its advantages are good exposure of portal vein and hepatic artery, feasibility of sharp cutting the hepatic plates around the hepatic duct, and ensuring the blood supply to hepatic duct and the ideal section.