and Andrea Bischoff1
(1)
Pediatric Surgery, Colorectal Center for Children Cincinnati Children’s Hospital, Cincinnati, OH, USA
22.1 Introduction
Anorectal malformations are represented by a wide spectrum of defects. On the good side of the spectrum, that includes, malformations that can be repaired with a relatively easy technique and can obtain excellent functional results, making the patient basically a normal individual that can enjoy a normal life. On the other hand, on the “bad side” of the spectrum, one can find complex defects, associated to very significant anatomic deficiencies that make the functional prognosis rather somber. In these serious and complex defects, it is almost impossible to restore normal bowel function, urinary function, or sexual function. Many of these patients are born without the necessary nerves and muscles that represent the mechanism of bowel and urinary control. In the middle of these two extremes of the spectrum, there are many types of malformation with variable anatomic setups and different prognoses. We look forward to the design and/or discovery of therapeutic methods that allow obtaining better functional results for patients that are currently born in the “bad side” of the spectrum.
However, it must be considered unacceptable to see a patient born with a malformation considered benign, in other words, in the “good side” of the spectrum to receive a technically deficient surgical procedure that destroys important structures and mechanisms of bowel and urinary control and ends up with a patient that belongs to the “bad side” of the spectrum. This is preventable and totally unacceptable. Unfortunately, it is something that happens more often than desired.
We do not know if there is a problem related with our perception, the fact that we feel that more morbidity occurs during the attempt to repair anorectal malformations than the one that occurs in other congenital defects. The fact is that at our center, from a total of 2,032 cases of anorectal malformations operated by the authors over the previous 30 years, 478 are reoperations. One hundred and fifty-three of those were done in an attempt to regain bowel control. Three hundred and twenty-five were done in cases that underwent an attempted failed repair at another hospital and suffered serious complications. We are aware of the fact that we are a referral center, and therefore the proportion of cases that we see, that had failed attempted repairs, is not representative of the proportion seen in the general population. Yet, we still consider this number extremely high. From a total of 909 male cases we had operated, 223 are reoperations, 93 of them were performed in an attempt to improve bowel control, and 130 were done to repair sequelae from injuries provoked by previous surgical misadventures. Between 1,123 female cases, 60 were reoperated in an attempt to regain bowel control and 195 for other reasons.
This takes us into a controversial issue of “reproducible” versus “non-reproducible” operations in surgery. We are convinced that some operations are highly reproducible, such as pyloromyotomy, hernia repair, cholecystectomy, and other similar procedures. Those operations have demonstrated the efficiency and efficacy through many years, performed by all kinds of surgeons. Unfortunately, we believe that the operations designed to repair anorectal malformations are not very reproducible. This means that to repair anorectal malformations successfully, the surgeon must be familiar with an anatomic territory that was not well known until recently. For that, he must be open-minded enough to forget many of the traditional anatomic concepts and become familiar with the anatomic spectrum seen in this complex part of the body, in patients with anorectal malformations. In addition, the surgeon in charge of repairing these defects must be very meticulous, careful, and delicate. There are, we are convinced, many surgeons who are very good to repair certain types of defects, but not for others. Many surgeons have a tendency to be in a hurry and do not have tolerance or patience to slow down when dealing with delicate tissues and complex anatomic arrangements. Anorectal malformation patients are born with a rectum, sometimes a vagina, and sometimes a urethra located in rather unusual places. These three structures are frequently abnormally attached one to another, and they share common walls without a plane of dissection. The separation of these structures is a mandatory step, in order to reconstruct the anatomy of these unfortunate patients. The separation of those structures represents a technical challenge. Until now, the only way to do it is observing a very meticulous, delicate dissection. This mandatory step (the separation of the structures) has not been facilitated by new technologic advances, such as laparoscopic approach or robotic approach. In fact, we have seen more complications when such separation has been attempted through a laparoscope. We are sure that new advances in the technology will result in finer, most likely digital instruments that will allow to perform complex reconstructions with minimally invasive type of procedures.
Professors of pediatric surgery, all over the world, are responsible for the surgical training of the young generation of pediatric surgeons that will be operating on thousands of babies born with these defects. We look forward to the time when all patients born with “benign” malformations undergo impeccable operations to repair the defect and enjoy a normal life.
Perhaps, part of the problem to explain why these operations are not as reproducible as others is the fact that the wide spectrum of anatomic variations found in these patients is not well known by the majority of pediatric surgeons. In addition, during the adult general surgical training of most surgeons, they learn the traditional anatomic concepts from adult general surgical textbooks, which are not representative of the anatomic variations seen in patients with anorectal malformations.
22.2 Reoperations to Improve Bowel Control
During our early experience with the posterior sagittal approach [1], we were extremely optimistic and believed that we would be able to restore the anatomy of many patients that have been operated with old techniques and suffered from fecal incontinence. We assumed that since most of the operations used before 1980 were performed, at least in part, blindly, one would expect that the surgeons may have positioned the rectum in the wrong location and not in the center of the sphincter mechanism. Based on that, we thought that by repositioning the rectum within the limits of the sphincter, the patient may gain bowel control. Consequently, we accepted to surgically reexplore all patients who underwent a previous repair and suffered from fecal incontinence. Our initial experience included eight patients [1]. In these, we found that the most common mislocation of the rectum was an anterior one (Fig. 22.1). These mislocations could be total or partial. The second most common mislocation of the rectum was a posterior one (Fig. 22.2). In general, the cases of posterior mislocated rectum were the patients operated at a time before the Stephens contribution, when surgeons believed that the rectum should be pulled down as close as possible to the sacrum to avoid damage to the urinary tract. The cases in whom the rectum was located too anterior, in general, belonged to the era when Dr. Stephens suggested that the rectum should be pulled down, as close as possible to the rectum, in order to preserve the “puborectalis sling” and give the patient the possibility of bowel control.
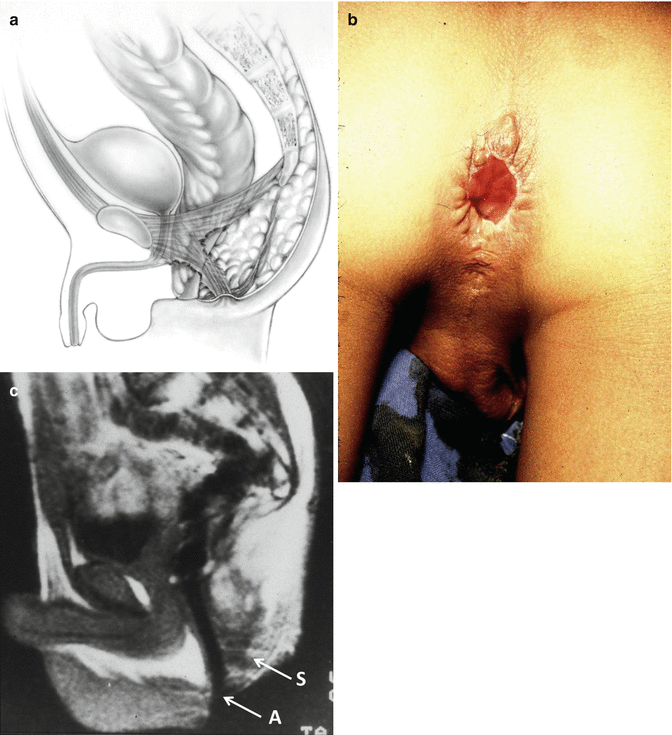
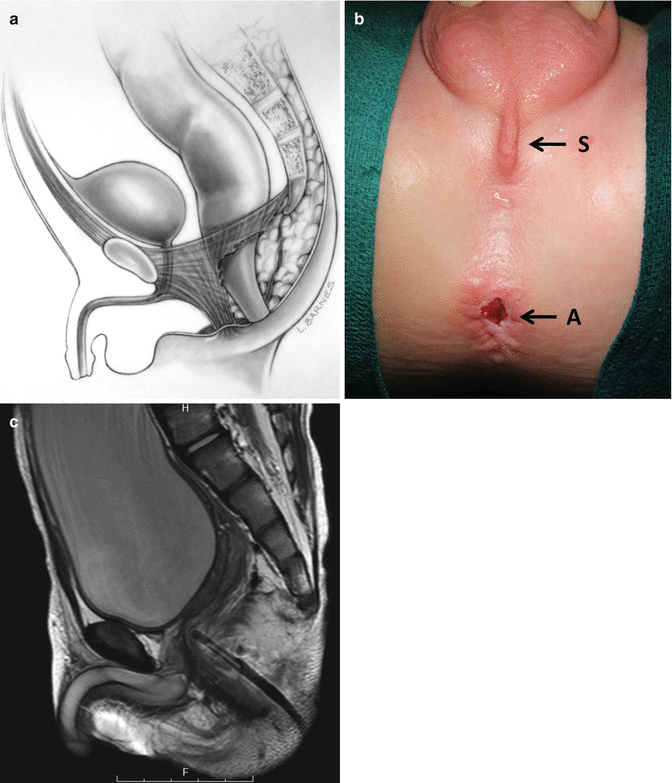

Fig. 22.1
Anterior mislocation of the rectum. (a) Diagram. (b) Perineum. (c) MRI. S sphincter, A anal opening

Fig. 22.2
Posterior mislocation of the rectum. (a) Diagram. (b) Perineum. S sphincter, A anal opening. (c) MRI
A third type of rectal mislocation was a lateral one that happened to be very unusual (Fig. 22.3).
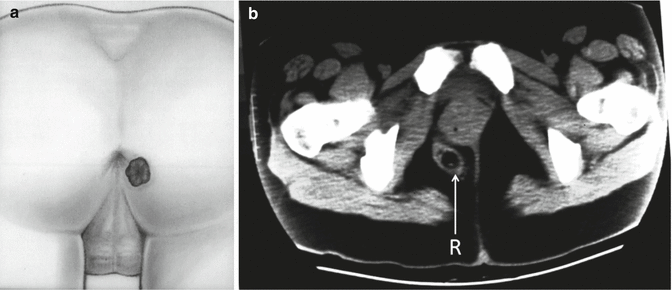

Fig. 22.3
Lateral mislocation of the rectum. (a) Diagram. (b) Perineum
The operation consists in opening posterior sagittally (Fig. 22.4). Multiple silk stitches are placed at the mucocutaneous junction of the anal opening in order to apply uniform traction to facilitate this redissection of the rectum. The incision is performed exactly in the midline, dividing all sphincter mechanisms found, posterior to the rectal wall. The incision continues until we identify the posterior rectal wall. Many times, what we really find is the colon. In other words, the surgeons who performed the first operation resected the rectum and pulled down colon from inside the abdomen. We must be prepared to be able to identify whether the patient has a colon or rectum.
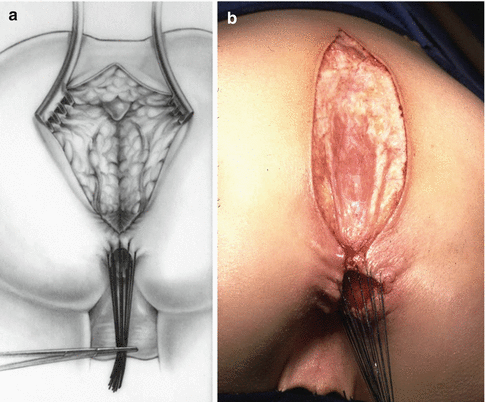

Fig. 22.4
Opening incision. (a) Diagram. (b) Operative
Originally, we were concerned and thought that this type of reoperation could be extremely difficult. Actually, it turned out that the reoperation was easier to perform than the primary procedures. Since most of these patients did not suffer from infections, retractions, and abscesses (catastrophes), the surgical planes and the anatomic features were easily recognized. In addition, it was not necessary to separate the rectum from the urogenital structures, which is, as we know, the most important challenge in the treatment of anorectal malformations. Also, there was no fistula, and finally, there was not a problem of bowel length, because the bowel was already connected to the perineum, so it turned out to be a rather quick and easy procedure.
Once we identify the posterior bowel wall, the dissection must be extended to the lateral walls of the bowel and eventually to the distal end at the skin. The posterior sagittal incision is continued in a circumferential manner, peripheral to the silk stitches to mobilize the entire rectum (Fig. 22.5).
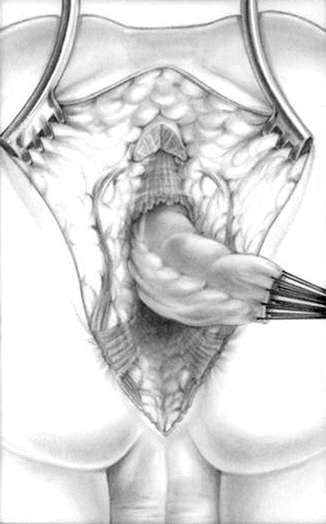

Fig. 22.5
Diagram showing the dissection of the rectum
Once the rectum has been mobilized, the limits of the sphincter are electrically determined (Fig. 22.6). Our findings in this type of operation include patients who had the rectum completely mislocated and an intact sphincter mechanism. In those procedures, it was extremely satisfactory to mobilize the rectum and place it within the limits of the sphincter. We finished the operation with the impression that we really benefited the patient. Other times, to our dismay, we found either that the patient was born with no sphincters and the rectum was surrounded by fat tissue or we found that the sphincter had been destroyed during the previous operation and the bowel was surrounded by scar tissue only. In those cases, we finished the procedure, feeling that we did not help the child.
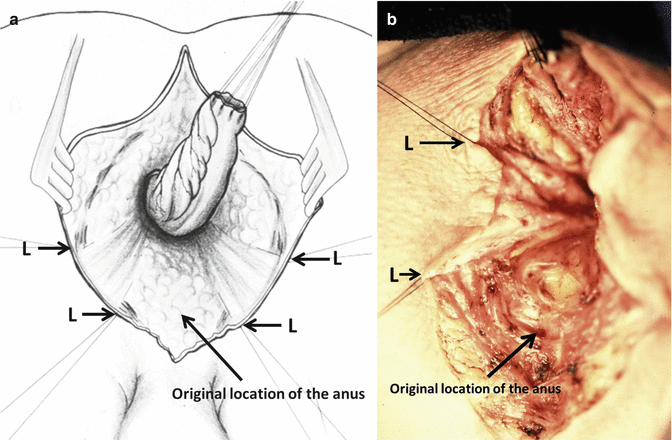

Fig. 22.6
Limits of the sphincter. (a) Diagram. L limits of the sphincter. (b) Operative view of one side of the incision, showing the limits of the sphincter and the original anterior mislocation of the anus
The rectum then is relocated and placed within the limits of the sphincter mechanism. The previous location of the rectum is obliterated and repaired with long-term absorbable sutures. The rectum is anchored to the sphincter mechanism as in the primary procedures, and the anoplasty is done with circumferential multiple, fine, long-term, absorbable sutures (Fig. 22.7).
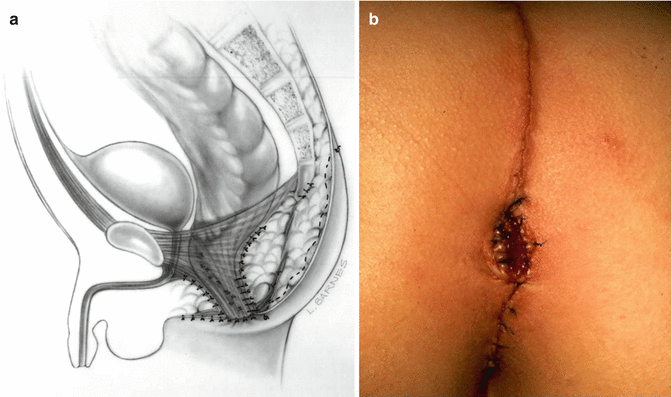

Fig. 22.7
Anoplasty. (a) Diagram. (b) Perineum
The first retrospective review of our experience with this type of operations showed us that five of our eight cases had a significant improvement in terms of bowel control [1]. In other words, they recovered voluntary bowel movements. We then analyzed the characteristics of the group of patients that improved with the operation and compared with the group that did not improve and found that, as expected, those patients who improved were the ones that had a completely mislocated rectum, a preserved rectum, and a good sacrum and were born with a malformation that belongs to the “good side” of the spectrum. Based on that experience, we changed the indications for this type of procedure, and ever since that time, we only reoperate, with the expectation to improve bowel control, on those patients that have a good sacrum (sacral ratio between 0.6 and 0.8), no evidence of tethered cord, and a completely mislocated rectum with an intact sphincter; they still have their original rectum (was not resected), and they were born with a malformation that we consider a “benign” type. The results of those reoperations with the new indications were not as good as we expected; only about 2/3 of our 77 patients improved significantly in terms of bowel control [2, 3]. Yet, 85 % of them still soil the underwear significantly.
The review of the history of the reoperations designed to improve bowel control, in patients suffering from fecal incontinence, is a demonstration of the ingenuity, imagination, and creativity of surgeons in general. However, the results have been in general less than optimal.
Those surgeons who believed in the existence of the puborectalis, soon enough, designed an operation to recover the “missed puborectalis” [4, 5]. Others [6–10] believed that they could improve bowel control by plicating, tightening, or releasing the levator mechanism from its posterior attachments. These types of procedures were known as “levatorplasties.”
A few surgeons embraced the idea of using the posterior sagittal approach to perform a relocation of the rectum or to perform a “levatorplasty” [11–16], most of them with encouraging results and others with bad results [12]. One author described an “anterior sagittal approach” to reoperate incontinent patients [17]. A careful reading of the article showed that the procedure was actually a posterior approach performed in lithotomy position.
Following the original idea of Pickrell [18], many surgeons tried the “gracilis sling” operation, with and without electrical stimulation, with questionable results [19–36]. The gluteus muscle was also used to create a voluntary sphincter [37–41] with variable results.
The possibility of using an artificial sphincter, capable of giving bowel control, has always been in the mind of surgeons [42–52]. It has been tried only in adult patients. The morbidity of this procedure is high, including infection, rectal stricture, and mechanical failure of the device. In 1988, we implanted in pigs Silastic, hydraulic devices normally used in the urinary tract (unpublished data). We were able to avoid bowel movements when the cuffs were inflated, but we were not able to produce bowel movements when the device was deactivated. That experience contributes to make us believe that we will not be able to produce bowel control, unless we find the way to manipulate the rectosigmoid motility.
In 1975, Hakelius from Sweden suggested that we could produce bowel control with free autogenous muscle transplantation [53, 54]. Several surgeons followed his idea, with variable results [55–59].
Surgeons who believed in the existence of the “internal sphincter” and its importance for bowel control devised ingenious procedures to create a structure similar to the “internal sphincter” [60–63]. The lack of long-term results makes us to suspect that the results have not been good.
Based on the idea that the contraction of the sphincter mechanism is a response to electric-like stimuli, transmitted by a nerve, some surgeons have been trying different modalities of electrical, magnetic, or temperature-controlled radiofrequency stimulation to produce bowel control. The reports describe mainly adult patients, and the results are rather controversial [64–74].
The extreme example of the rather simplistic and naïve idea of the mechanisms of bowel control is the use of a tampon-like device [75] or by the injection of bulking agents in the anus [76, 77].
In summary, we believe that a rational approach to the problem of fecal incontinence in children must consist in the regulation of colonic motility in patients with borderline fecal incontinence. In other words, patients with mild degree of incontinence may benefit by the use of medication and/or diet to slow down the colon, in cases with tendency to diarrhea and the use of laxatives in those patients who suffer from constipation.
In some cases with total fecal incontinence consecutive to a previously repaired anorectal malformation, we offer a reoperation to those patients that have the following characteristics:
A.
Completely mislocated rectum
B.
Have normal sacrum
C.
No evidence of tethered cord
D.
Were born with good prognosis type of anorectal malformation (perineal fistula, vestibular fistula, bulbar fistula, and absent fistula)
In all other cases, or those who did not respond to our procedure, we offer them our bowel management program (see Chap. 20).
22.3 Reoperations Performed After Failed Attempted Repair (Catastrophes) Males
We have done approximately 130 cases of male patients born with an anorectal malformation, who underwent an attempted failed repair. These patients suffered from postoperative acquired rectal atresia, anorectal stenosis, dehiscence, retractions, abscess, infections, and persistent, recurrent, or acquired rectourethral fistulas.
Interestingly, in all these cases, we found that the common denominator to explain the failed operation was the lack of a preoperative high-pressure distal colostogram or a technically deficient one. The surgeons did not have accurate anatomic information, which resulted in a deficient surgical technique.
The lack of a high-pressure distal colostogram frequently induced the surgeons to look for the rectum in the wrong place, damaging other important structures, including the urethra, vas deferens, and seminal vesicles, or provoking nerve damage that resulted in neurogenic bladder and/or lack of erections (impotence). The most common scenario was a patient with anorectal malformation with a recto-bladder neck fistula or a very high rectal prostatic fistula that was approached posterior sagittally without a distal colostogram. The surgeon went straight deep through the incision, looking for the rectum that was not there, and in the process of searching for the rectum, he injured the structures that we already mentioned.
Another scenario was a surgeon who entered posterior sagittally, without a distal colostogram, found the rectum, separated it from the urinary tract, and tried to mobilize it down unsuccessfully. The surgeon was unaware of the fact that the patient had a very short piece of bowel from the mucous fistula of the colostomy to the distal end or fistula of the rectum (see Chap. 5). The fact that the patient did not have a distal colostogram did not allow the surgeon to predict that he would find such anatomic limitation. As a consequence, after persistent, unsuccessful attempts to mobilize the rectum, the result usually was devascularization of the bowel. The surgeons had to go into the abdomen, resect that damaged piece of bowel, and take the proximal colostomy down. This had important negative repercussions for the patient in terms of bowel control, because we have evidence that when the original rectum is resected, the chances for the patient to have bowel control are significantly decreased.
Other cases had an unnecessarily extensive operation for a rather benign defect. This happened when a patient with a benign malformation (such as perineal fistula) underwent a non-indicated colostomy and subsequently a technically deficient distal colostogram. The study was done without applying the necessary hydrostatic pressure in order to distend the most distal part of the bowel, which we know is surrounded by voluntary sphincter mechanism (see Chap. 6, Sect. 6.6). The technically deficient distal colostogram showed an image that gave the wrong impression; the surgeon and the radiologists believed that the patient was born with “high imperforate anus.” Actually the patient had a rectum located much lower and could be easily reached with an incision from below (posterior sagittally). Rather than that, the surgeon went ahead and performed a laparotomy or laparoscopy, which was not indicated. Trying to separate the rectum from the urinary tract, through the abdomen, with a laparotomy or laparoscopy in a case of a very low-lying rectum is a very difficult task, and occasionally the patients suffered a urethral damage or were left with a piece of rectum attached to the urethra (posterior urethral diverticulum).
22.4 Reoperations for Postoperative Recto-urinary Fistula
Fifty-two cases came to us, with a fistula that connected the rectum to the urinary tract, with a history of a previous failed attempted repair. This condition has been reported in the past [78–87]. We classified those fistulas into three categories:
22.4.1 Recurrent Fistula (17 Cases)
In these cases, the surgeon recognized the presence of the fistula, separated the rectum from the urinary tract, closed the fistula during the operation, and then repaired the anorectal malformation, and yet the patient developed a recurrent fistula (Fig. 22.8a, b). A careful and detailed reading of the operative report of those cases, as well as our findings in the reoperations, leads us to believe that the main problem in these type of cases was the fact that during the separation of the rectum from the urinary tract, the anterior rectal wall was damaged more than necessary, as well as the posterior wall of the urethra next to the fistula site. In addition, the rectum was not mobilized enough, and most likely an anterior rectal wall, already damaged, was left located in front of a sutured-damaged urethra. As we know, leaving sutures, in front of sutures, represent the ideal situation for the recurrence of a fistula.
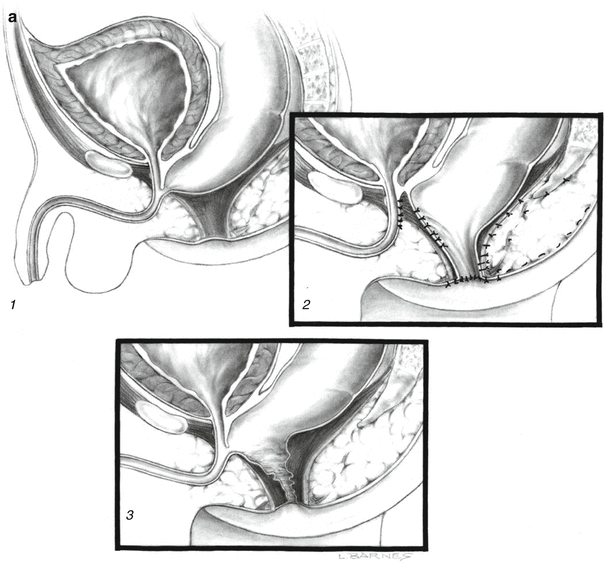
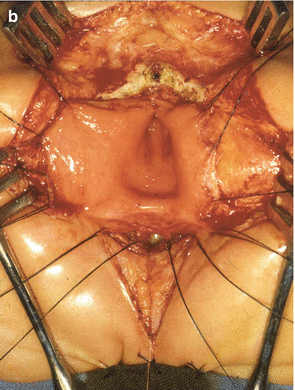


Fig. 22.8
Recurrent fistula. (a) Diagram. (1) Preoperative. (2) Postoperative. The rectum was not mobilized enough; sutures of the rectum were left in front of urethral sutures. (3) Recurrent fistula. (b) Intraoperative picture showing the recurrent fistula
Our reoperation consisted of a posterior sagittal approach, separation of the rectum from the urinary tract, which was more difficult than the original procedure and then performed enough mobilization of the rectum, so as to be sure that we left a normal rectal wall in front of a urethral suture.
22.4.2 Persistent Rectourethral Fistula (24 Cases)
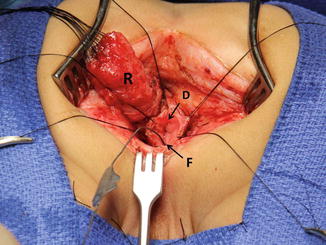
This occurs when the patient was born with a rectourethral fistula, most of the time located in the lowest part of the posterior urethra (bulbar) (Fig. 22.9a, b). In these types of cases, the lowest part of the rectum bulges down and is seen in the radiologic diagnostic studies as “low” malformation. However, the radiologist did not apply enough hydrostatic pressure to demonstrate the fistula. As a consequence, the surgeon believes that he is dealing with a low-lying rectum without a fistula that he can approach from below. Consequently, he enters through a posterior sagittal or perineal approach, finds the rectum relatively easily, mobilizes only the necessary part of the rectum in order to create an anus, and finishes the operation leaving the rectourethral fistula untouched and intact (Fig. 22.9).
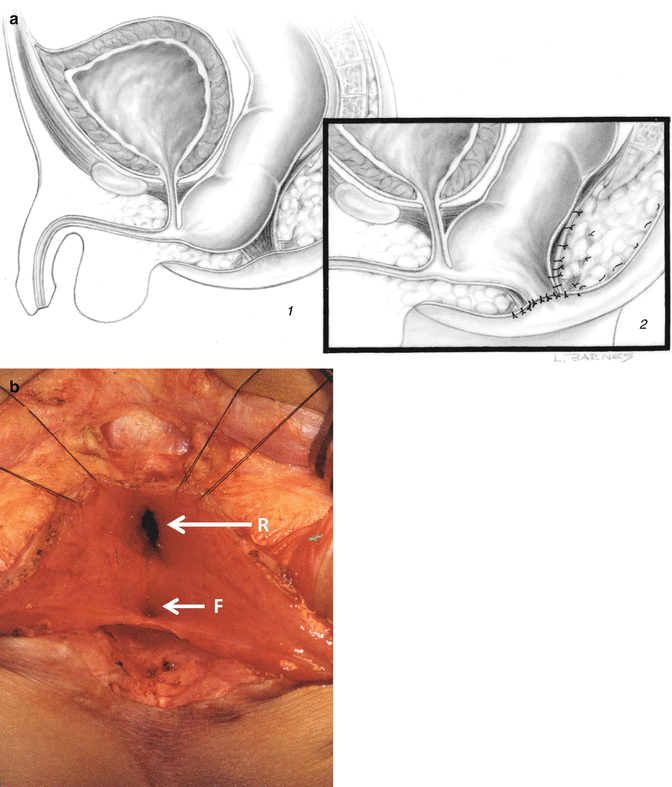

Fig. 22.9
Persistent fistula. (a) Diagram. (1) Preoperative. (2) Postoperative – the rectum was pulled down, and the fistula was left intact. (b) Intraoperative – picture taken during the posterior sagittal approach. R rectum, F fistula
The reoperation in this type of case is straightforward. First, we go posterior sagittally, open the rectum like we do in a primary procedure, and deal with the fistula exactly in the same way as we do in a primary procedure. Again, we must be certain to leave a completely normal rectal wall in front of the urethral fistula site.
22.4.3 Acquired Fistula (9 Cases)
In these cases, the patients were born with an anorectal malformation, without a fistula, and underwent an operation, and after the procedure, they passed urine through the rectum and stool through the urethra! Obviously, the fistula was created by the surgeon. The most common scenario is the one of a patient who was born with a perineal fistula and underwent an operation; the surgeon did not use a Foley catheter in the urethra and damaged or divided inadvertently the urethra during the mobilization of the anterior rectal wall (Fig. 22.10).
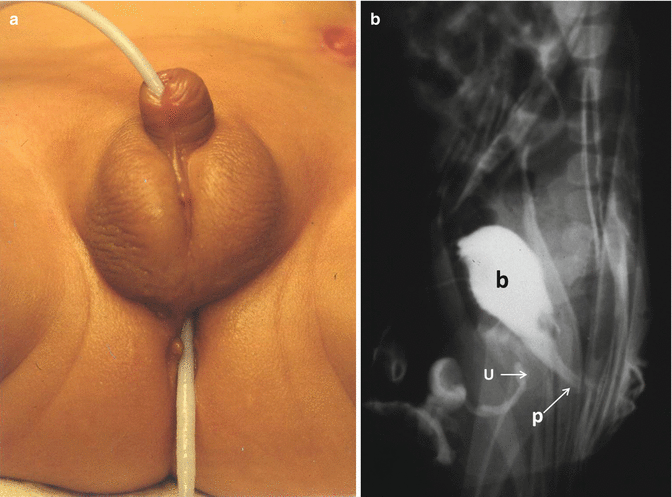

Fig. 22.10
Acquired fistula. (a) Foley catheter passing through the penis and coming out through the anus. (b) Cystogram of a patient born with a perineal fistula, operated without a Foley catheter. The urethra was completely transected. b bladder, u urethral blind end, p proximal urethral end pulled down together with the rectum
The operation in these cases consists of separating the rectum from the perineum and approach the urethra to close the fistula. Then mobilize the rectum again to be sure to leave a normal rectal wall in front of the urethra. These types of patients frequently have a complete section of the urethra and require a resection of the stricture and reanastomosis, which can be done comfortably posterior sagittally with good results.
22.5 Posterior Urethral Diverticulum (32 Cases)
We use this term to refer to patients who underwent a repair of an anorectal malformation and were left with a piece of rectum attached to the urethra (Fig. 22.11) [83–88]. That piece of rectum behaves like a diverticulum (see Chap. 23). It has negative implications, including urinary pseudoincontinence, formation of stones, urinary tract infections, and orchiepididymitis, and in one particular case, the patient developed an adenocarcinoma at the junction of the piece of the rectum with the urethra.