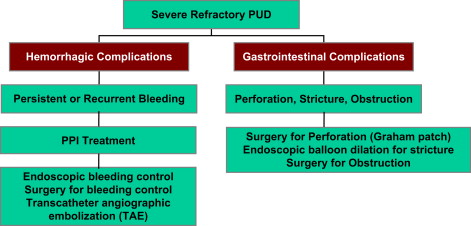
Refractory peptic ulcer disease (PUD) manifests as either hemorrhagic complications (persistent or recurrent bleeding) or gastrointestinal (GI) complications (perforation, stricture, obstruction). Treatment strategies for hemorrhagic complications include Endoscopic therapy, surgery, and transcatheter angiographic embolization. Treatment strategies for GI complications include endoscopic dilation for stricture and surgery for perforation and obstruction. Potential etiologies of persistent or worsening PUD must be considered in these cases and include the following: patient risk factors and noncompliance, persistent Helicobacter pylori infection, and non– H pylori– related infection, related to underlying idiopathic gastric hypersecretion or Zollinger-Ellison syndrome and gastrinoma. An appropriate and meticulous diagnostic work-up for refractory PUD is mandatory.
Although the incidence of peptic ulcer disease (PUD) in Western countries has declined over the past 100 years, about 1 in 10 Americans are still affected. As the prevalence of PUD increased with advancing age, it is expected that this common disease will continue to have a significant global impact on health care delivery, health economics, and the quality of life of patients.
PUD is the main cause for upper gastrointestinal (UGI) hemorrhage, and Helicobacter pylori infection is the main etiologic factor for PUD. Medical regimens to identify and eradicate the organism and the widespread use of proton pump inhibitor (PPI) therapy to suppress gastric acid secretion have resulted in successful medical management of PUD in the vast majority of patients. As a result, successful medical management of PUD has largely supplanted the need for gastric surgery by general surgeons.
Surgery of PUD is now limited to treatment of more emergent complications of the disease (hemorrhage, perforation, gastric outlet obstruction), refractory disease and intractability (related to bleeding or gastrointestinal [GI] complications), or rare causes of ulcer disease, such as gastrinoma and the Zollinger-Ellison syndrome (ZES). Indications for elective peptic ulcer surgery include the following: resection of ulcers suspicious for malignancy, failure to heal despite maximal medical therapy, intolerance or noncompliance with medical therapy, and relapse while on maximal medical therapy.
In this article diagnostic and treatment issues related to refractory PUD are reviewed. It is most important to ensure that appropriate standard therapy for PUD is provided with subsequent confirmation of eradication of H pylori infection, because this is the best method for prevention of refractory PUD. If refractory PUD does occur, it is important to have a systematic approach for diagnosis and treatment. Refractory PUD manifests as either hemorrhagic complications (persistent or recurrent bleeding) or GI complications (perforation, stricture, obstruction). Treatment strategies for hemorrhagic complications include endoscopic therapy, surgery, and transcatheter angiographic embolization. Treatment strategies for GI complications include endoscopic dilation for stricture and surgery for perforation and obstruction. Potential etiologies of persistent or worsening PUD must be considered in these cases and include the following: patient risk factors and noncompliance, persistent H pylori infection, and non– H pylori –related infection, related to underlying idiopathic gastric hypersecretion or ZES and gastrinoma. An appropriate and meticulous diagnostic work-up for refractory PUD is mandatory.
Standard therapy for Peptic Ulcer Disease
The widespread use of effective antisecretory therapies, including PPIs, and the recognition and successful eradication of H pylori infection have made peptic ulcer a disease that can be cured by medical management in most cases. Surgical intervention had once been the dominant form of definitive therapy, but it is now reserved for emergent, life-threatening complications of PUD, such as bleeding, perforation, and obstruction. Intractability, failure to comply with or tolerate medical therapy, and rare cases of gastrinoma or ZES are indications for elective surgery for PUD.
H pylori is associated with 95% of duodenal ulcers and 70% of gastric ulcers, and eradication of H pylori reduces the relapse rate of ulcers. The 2004 Cochrane evidence-based review of 53 randomized controlled trials of short- and long-term treatment of PUD in H pylori -positive adults examined the effect of this treatment. Patients received at least 1 week of H pylori eradication therapy compared with ulcer-healing drug, placebo, or no treatment. In duodenal ulcer healing, H pylori eradication therapy was superior to ulcer-healing drug (34 trials, 3910 patients, relative risk [RR] of ulcer persistence, 0.66; 95% confidence interval [CI], 0.58–0.76) and no treatment (two trials, 207 patients, RR, 0.37; 95% CI, 0.26–0.53). In gastric ulcer healing, no significant differences were detected between eradication therapy and ulcer-healing drug (13 trials, 1469 patients, RR, 1.32; 95% CI, 0.92–1.90). This confirmed that a 1- to 2-week course of H pylori eradication therapy is an effective treatment for H pylori -positive PUD.
There is now a worldwide consensus that the first-line treatment of H pylori infection should be triple therapy with a PPI twice daily plus clarithromycin 500 mg twice daily and either amoxicillin 1 g twice daily or metronidazole 500 mg twice daily for 7 to 14 days. Treatment with PPIs twice daily is superior to treatment once daily. Bismuth-containing quadruple therapy, if available, is also a first choice treatment option. Successful eradication with first-line treatments varies from 70% to 95%, and 10- and 14-day treatments are generally 7% to 9% more effective than the most commonly used 7-day regimens. Rescue treatment should be based on antimicrobial susceptibility.
Eradication of H pylori infection should be confirmed after the completion of therapy and noninvasive testing with the urea breath test is the preferred choice, 4 to 8 weeks after the completion of therapy ( Table 1 ). If the ulcer recurs after the eradication therapy, a more careful search for reinfection or eradication failure should be performed by testing for the presence of active infection (by histologic examination and culture, together with an antibiotic-sensitivity test). The diagnosis of H pylori infection in patients with a bleeding PUD is limited by the decreased sensitivity of standard invasive tests; usually, both the rapid urease test and histologic testing should be performed during endoscopy and then combined with the urea breath test. Infection should be considered as present when any test is positive, whereas the invasive tests and the urea breath test should be negative to establish the absence of H pylori infection.
| Diagnostic Test | Specific Issues | Can Be Used to Confirm Eradication |
|---|---|---|
| Serologic ELISA |
| No |
| Urea breath test |
|
|
| Stool antigen test |
| Yes |
| Urine-based ELISA and rapid urine test |
| No |
| Endoscopic biopsy |
| Yes |
Furthermore, it is well accepted that in patients with uncomplicated PUD, H pylori eradication therapy need not be followed by antisecretory treatment. A 5-year prospective controlled study randomized 82 patients with H pylori -associated bleeding peptic ulcers to 1 of 4 16-week maintenance treatment groups after successful H pylori eradication with a 1-week PPI-based triple therapy and an additional 43-week treatment with 20 mg of omeprazole daily for ulcer healing. The four experimental groups were as follows: group A received 15 mL of an antacid suspension four times daily; group B received 300 mg of colloidal bismuth subcitrate four times daily, group C received 20 mg of famotidine twice daily; and group D, the control group, received placebo twice daily. Follow-up included a urea breath test labeled with carbon 13, biopsy-based tests, and repeated endoscopic examination. During a mean follow-up of 56 months, there was no peptic ulcer recurrence among the three treatment groups, and all the patients remained free of H pylori infection during the study period. This study documented that in patients with bleeding peptic ulcers, antiulcer maintenance treatment was not necessary to prevent ulcer recurrence after successful H pylori eradication and ulcer healing. Besides, the 1-week PPI-based triple therapy had the efficacy to ensure long-term eradication of H pylori in a region of high prevalence.
Refractory Peptic Ulcer Disease
Refractory PUD is defined as a disease that fails to heal after 8 to 12 weeks of therapy or one that is associated with complications. It is most challenging to evaluate and treat patients with complicated and/or refractory PUD. A recent analysis regarding admission rates for PUD in the United Kingdom during 1972 to 2000 determined that emergency admission rates as a whole changed little, a decline in the young being offset by an increase in the elderly. Hemorrhage was the most common reason (approximately 115 per million population for duodenal ulcer and 87 for gastric ulcer) throughout (compared with perforation [80 and 21] and pain [90 and 68]).
Refractory Peptic Ulcer Disease and Bleeding
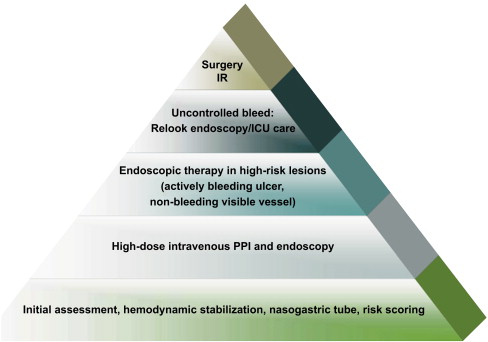
Acute UGI bleeding related to refractory PUD remains a challenging clinical problem owing to significant patient morbidity and mortality. PUD accounts for 28% to 59% of all episodes of UGI bleeding. The mortality rate associated with bleeding duodenal ulcer disease is about 10%. The first priority in treatment of bleeding due to refractory PUD is the initiation of resuscitation, critical care support, and PPI therapy ( Fig. 1 ). A systematic review of the clinical efficacy of PPI in acute UGI bleeding concluded that PPI treatment compared with placebo or histamine-2 receptor antagonists (H2RAs) reduces mortality following PUD bleeding among patients with high-risk endoscopic findings, and reduces hemorrhage recurrence rates and surgical intervention. PPI treatment initiated before endoscopy in UGI bleeding significantly reduced the proportion of patients with stigmata of recent hemorrhage (SRH) at index endoscopy but did not reduce mortality, rebleeding, or the need for surgery in this analysis. More recently, the initiation of PPI bolus followed by continuous infusion after endoscopic therapy in patients with bleeding ulcers significantly improved outcome compared with placebo/no therapy (RR, 0.40, 95% CI, 0.28–0.59; number needed to treat [NNT], 12, 95% CI, 10–18), but not compared with H2RA. The strategy of giving PPI before and after endoscopy, with endoscopic hemostatic therapy for those with major SRH, is the most cost-effective. Treatment of H pylori infection was found to be more effective than antisecretory therapy in preventing recurrent bleeding from PUD. Further large randomized controlled trials are needed to address areas, such as PPI administration before endoscopic diagnosis, different doses and administration of PPIs, as well as the primary and secondary prevention of UGI bleeding.
Endoscopy is the preferred first-line management of refractory bleeding due to PUD. Current endoscopic modalities, both thermal and nonthermal, offer a wide range of choices in high-risk PUD bleeding (active arterial bleeding or nonbleeding visible vessel). Combinations of injection (epinephrine) along with thermal therapy or endoclips are recommended for better clinical outcomes. A recent review concluded that all endoscopic treatments are superior to pharmacotherapy alone in peptic ulcer bleeding. Optimal endoscopic therapies include thermal therapy or clips, either alone or in combination with other methods, but epinephrine injection should not be used alone. The role of endotherapy for adherent clots is controversial. A second-look endoscopy may be beneficial in high-risk patients.
Primary endoscopic hemostasis is successful in more than 90% of patients, but in 15% to 25% of the patients, either the bleeding cannot be controlled endoscopically or there is recurrence of bleeding, requiring alternative treatment. The combination of endoscopic intervention for hemostasis and PPI therapy is necessary to achieve hemostasis of active bleeding related to PUD. Continued bleeding after attempted endoscopic control may warrant surgical intervention. A multidisciplinary team approach should be part of all treatment protocols for the ideal management of refractory UGI hemorrhage related to PUD, and early surgical consultation is required.
An emerging strategy for bleeding control in refractory PUD is angiographic embolization (see Fig. 1 ). In patients who are poor surgical candidates because of their high operative risk, percutaneous transcatheter angiographic arterial embolization (TAE) is a therapeutic option. A recent study evaluated the efficacy and medium-term outcomes of TAE to control massive bleeding from gastroduodenal ulcers after failed endoscopic treatment in high-operative-risk patients. This was a retrospective study of 35 consecutive emergency embolization procedures in hemodynamically unstable patients (24 men, 11 women, mean age 71±11.6 y) referred from 1999 to 2006 for selective angiography after failed endoscopic treatment. Mean follow-up was 27 months. Endovascular treatment was feasible in 33 patients and consistently stopped the bleeding. “Sandwich” coiling of the gastroduodenal artery was performed in 11 patients and superselective occlusion of the terminal-feeding artery with glue, coils, or gelatin particles in 22 patients. Early rebleeding occurred in six patients and was managed successfully using endoscopy (n = 2), reembolization (n = 1), or surgery (n = 3). No major complications related to TAE occurred. Seven patients died within 30 days of TAE and three died later during the follow-up, but none of the deaths were due to rebleeding. No late bleeding recurrences were reported. These investigators concluded that selective TAE is safe and effective for controlling life-threatening bleeding from gastroduodenal ulcers, usually obviating the need for emergency surgery in critically ill patients, whose immediate survival depends on their underlying conditions.
Previous reports also evaluated the efficacy and safety of TAE. In a 6-year review of 40 consecutive patients with bleeding/rebleeding after endoscopic therapy and/or surgery for duodenal ulcer, superselective angiographic catheterization and coil embolization were performed by the same interventional radiologist. Lasting hemostasis was achieved in 26 of 40 patients (65%). Transfusion requirement was reduced from median 14 (range, 3–35) units of blood before TAE to two (range, 0–53) units after TAE. Ten patients died, half of them because of continuous bleeding. No adverse effects as a result of TAE were observed.
A recent retrospective review identified all patients admitted to Ullevål University Hospital with hematemesis and/or melena and endoscopically verified duodenal ulcer from June 2000 to 2005. The indication for TAE was endoscopically unmanageable bleeding/rebleeding or rebleeding after surgery. Technical success was defined as acute hemostasis. Clinical success was defined as technical success without rebleeding within 30 days. A total of 278 patients (mean age, 73 years) were included in the study. Primary endoscopic hemostasis failed in 13 patients (5%) and 53 patients (20%) experienced rebleeding. An attempt was made to treat 36 patients with TAE. Technical success in the TAE group was 92% and clinical success was 72%. In total, 10 patients underwent surgery, three because of rebleeding after TAE. The 30-day mortality was 10% for all patients, 19% in the TAE group, and 20% in the surgical group. High technical and clinical success was obtained with TAE in patients with bleeding duodenal ulcer after failure of endoscopic treatment in this cohort study.
A retrospective review of the outcome of TAE and surgery as salvage therapy of UGI bleeding after failed endoscopic treatment was recently performed in 658 patients referred for diagnostic/therapeutic emergency endoscopy and diagnosed with UGI bleeding (January 1998–December 2005). Of these 658 patients, 91 (14%) had repeat bleeding or continued to bleed. Forty of those 91 patients were treated with TAE and 51 were underwent surgery. Patients treated with TAE were older (mean age, 76 years; age range, 40–94 years) and had slightly more comorbidities compared with patients who underwent surgery (mean age, 71 years; age range, 45–89 years). The 30-day mortality rate in patients treated with TAE was 1 of 40 (3%) compared with 7 of 51 (14%) in patients who underwent surgery ( P <.07). Most repeat bleeding could be effectively treated with TAE, both in the surgical and TAE groups. The results of this study suggest that, after failure of therapeutic endoscopy for UGI bleeding, TAE should be the treatment of choice before surgery and that TAE can also be used to effectively control bleeding after failed surgery or TAE. There was a clear trend to lower 30-day mortality with the use of TAE instead of surgery.
The data from these cohort studies document that TAE is an effective and safe treatment in a significant proportion of patients with bleeding/rebleeding duodenal ulcers after therapeutic endoscopy and/or surgery and may serve as an alternative to surgery in high-risk patients ( Fig. 2 ).
Refractory Peptic Ulcer Disease and Gastrointestinal Complications
GI complications related to refractory PUD include perforation (duodenal or gastric perforation) and obstruction, either partial or complete gastric outlet obstruction related to stenosis and stricture at the ulcer site. These GI complications can be challenging to treat and frequently require surgical intervention.
Perforation related to Peptic Ulcer Disease
Perforation occurs in approximately 2% to 10% of patients with PUD. It usually involves the anterior wall of the duodenum (60%), although it may also occur in antral (20%) and lesser-curve (20%) gastric ulcers. Recent data strongly implicate H pylori infection as the cause of perforated duodenal ulcer, with reported H pylori infection rates of 70% to 92% in these patients. A randomized study in 129 patients with duodenal ulcer perforation documented that 104 (81%) were infected with H pylori , diagnosed by esophagogastroduodenoscopy and biopsy at the time of laparotomy. Postoperatively, patients were randomized to receive H pylori treatment or PPI therapy for 4 weeks. Repeat endoscopy at 1 year confirmed that the incidence of recurrent ulceration was significantly lower in the H pylori treatment group (5%) compared with the PPI therapy group (38%). Based on these findings, surgical treatment for perforated duodenal ulcer is simple patch closure with postoperative H pylori treatment, including PPI therapy and antimicrobial agents, and documentation of eradication. Some patients with complicated perforated ulcer, either with destruction of proximal duodenum and penetration into adjacent organs, giant perforations measuring more than 20 mm in diameter or with severe duodenal stenosis, may require resectional surgery.
Perforated duodenal ulcer with perforation free into the peritoneal cavity is associated with peritonitis and warrants emergency surgical intervention. Both conventional laparotomy and laparoscopic techniques for suture closure with omental patch are acceptable surgical options for treatment in these patients. A randomized clinical trial (n = 130) did identify that laparoscopic repair of perforated PUD was associated with a shorter operating time, less postoperative pain, reduced pulmonary complications, shorter postoperative hospital stay, and earlier return to normal daily activities compared with the conventional open surgery, but surgeon’s laparoscopic experience and severity of illness of the patient must be considered in this decision making. A Cochrane Systematic Review concluded that laparoscopic surgery results are not clinically different from those of open surgery in patients with perforated PUD. Another systematic review concluded that laparoscopic repair seemed better than open surgery for low-risk patients, and that limited knowledge about its benefits and risks compared with open surgery suggests that the open approach may be more appropriate in high-risk studies. A more recent small prospective cohort study (n = 33) suggested that laparoscopic repair should be considered for all patients provided the necessary expertise is available. Specific factors have been identified that qualify as criteria for open laparotomy, including shock, delayed presentation—for more than 24 hours, confounding medical conditions, age more than 70 years, poor laparoscopic expertise, and American Society of Anesthesiologists score III to IV. However, additional studies are warranted in this area.
Obstruction related to Peptic Ulcer Disease
In patients presenting with gastric outlet obstruction, PUD is the underlying cause in up to 8% of patients. Many of them, however, have refractory PUD related to recurrent or persistent duodenal or pyloric channel ulcers that evolve into pyloric stenosis and obstruction as a result of acute and chronic inflammation, spasm, edema, scarring, and fibrosis. Initial management includes nasogastric decompression, antisecretory therapy, and eradication of H pylori . Endoscopic evaluation is necessary to determine the site, cause, and degree of obstruction and to evaluate for carcinoma as an etiology of the obstruction, because malignancy is the most common cause of gastric outlet obstruction in this era of antisecretory therapy.
Treatment of gastric outlet obstruction related to refractory PUD includes endoscopic pyloric balloon dilation and surgery. Endoscopic balloon dilation has been used for treatment of gastric outlet obstruction with variable results. Several large studies have demonstrated high rates of success for the relief of symptoms from pyloric stenosis using balloon dilation, which increases the diameter of the stenotic pylorus on average from 6 to 16 mm. Patients who require more than two dilations are at a high risk of endoscopic failure and the need for surgical intervention. Because many patients with benign pyloric stenosis have underlying ulcer disease, H pylori infection is a common finding. Eradication of this infection at the time of balloon dilation will ensure higher long-term success rates. Endoscopic balloon dilation should therefore be the first-line therapy in appropriate patients with benign pyloric stenosis related to PUD.
Obstruction necessitates operation in about 2000 patients per year in the United States. Surgical procedures that are considered in gastric outlet obstruction related to refractory PUD include vagotomy and pyloroplasty, antrectomy, and gastroenterostomy. Minimally invasive laparoscopic techniques (truncal vagotomy, gastrojejunostomy) have been developed for some of these surgical procedures that are associated with reduced postoperative recovery time. The largest series of laparoscopic procedures for the management of refractory PUD included 263 patients who were treated for either refractory PUD or obstruction due to PUD. Laparoscopic posterior truncal vagotomy with anterior proximal gastric vagotomy for refractory disease and laparoscopic bilateral truncal vagotomy with stapled gastrojejunostomy for obstructive disease have become the standard surgical management at this institution.
Refractory Peptic Ulcer Disease
Refractory PUD is defined as a disease that fails to heal after 8 to 12 weeks of therapy or one that is associated with complications. It is most challenging to evaluate and treat patients with complicated and/or refractory PUD. A recent analysis regarding admission rates for PUD in the United Kingdom during 1972 to 2000 determined that emergency admission rates as a whole changed little, a decline in the young being offset by an increase in the elderly. Hemorrhage was the most common reason (approximately 115 per million population for duodenal ulcer and 87 for gastric ulcer) throughout (compared with perforation [80 and 21] and pain [90 and 68]).
Refractory Peptic Ulcer Disease and Bleeding
Acute UGI bleeding related to refractory PUD remains a challenging clinical problem owing to significant patient morbidity and mortality. PUD accounts for 28% to 59% of all episodes of UGI bleeding. The mortality rate associated with bleeding duodenal ulcer disease is about 10%. The first priority in treatment of bleeding due to refractory PUD is the initiation of resuscitation, critical care support, and PPI therapy ( Fig. 1 ). A systematic review of the clinical efficacy of PPI in acute UGI bleeding concluded that PPI treatment compared with placebo or histamine-2 receptor antagonists (H2RAs) reduces mortality following PUD bleeding among patients with high-risk endoscopic findings, and reduces hemorrhage recurrence rates and surgical intervention. PPI treatment initiated before endoscopy in UGI bleeding significantly reduced the proportion of patients with stigmata of recent hemorrhage (SRH) at index endoscopy but did not reduce mortality, rebleeding, or the need for surgery in this analysis. More recently, the initiation of PPI bolus followed by continuous infusion after endoscopic therapy in patients with bleeding ulcers significantly improved outcome compared with placebo/no therapy (RR, 0.40, 95% CI, 0.28–0.59; number needed to treat [NNT], 12, 95% CI, 10–18), but not compared with H2RA. The strategy of giving PPI before and after endoscopy, with endoscopic hemostatic therapy for those with major SRH, is the most cost-effective. Treatment of H pylori infection was found to be more effective than antisecretory therapy in preventing recurrent bleeding from PUD. Further large randomized controlled trials are needed to address areas, such as PPI administration before endoscopic diagnosis, different doses and administration of PPIs, as well as the primary and secondary prevention of UGI bleeding.
Endoscopy is the preferred first-line management of refractory bleeding due to PUD. Current endoscopic modalities, both thermal and nonthermal, offer a wide range of choices in high-risk PUD bleeding (active arterial bleeding or nonbleeding visible vessel). Combinations of injection (epinephrine) along with thermal therapy or endoclips are recommended for better clinical outcomes. A recent review concluded that all endoscopic treatments are superior to pharmacotherapy alone in peptic ulcer bleeding. Optimal endoscopic therapies include thermal therapy or clips, either alone or in combination with other methods, but epinephrine injection should not be used alone. The role of endotherapy for adherent clots is controversial. A second-look endoscopy may be beneficial in high-risk patients.
Primary endoscopic hemostasis is successful in more than 90% of patients, but in 15% to 25% of the patients, either the bleeding cannot be controlled endoscopically or there is recurrence of bleeding, requiring alternative treatment. The combination of endoscopic intervention for hemostasis and PPI therapy is necessary to achieve hemostasis of active bleeding related to PUD. Continued bleeding after attempted endoscopic control may warrant surgical intervention. A multidisciplinary team approach should be part of all treatment protocols for the ideal management of refractory UGI hemorrhage related to PUD, and early surgical consultation is required.
An emerging strategy for bleeding control in refractory PUD is angiographic embolization (see Fig. 1 ). In patients who are poor surgical candidates because of their high operative risk, percutaneous transcatheter angiographic arterial embolization (TAE) is a therapeutic option. A recent study evaluated the efficacy and medium-term outcomes of TAE to control massive bleeding from gastroduodenal ulcers after failed endoscopic treatment in high-operative-risk patients. This was a retrospective study of 35 consecutive emergency embolization procedures in hemodynamically unstable patients (24 men, 11 women, mean age 71±11.6 y) referred from 1999 to 2006 for selective angiography after failed endoscopic treatment. Mean follow-up was 27 months. Endovascular treatment was feasible in 33 patients and consistently stopped the bleeding. “Sandwich” coiling of the gastroduodenal artery was performed in 11 patients and superselective occlusion of the terminal-feeding artery with glue, coils, or gelatin particles in 22 patients. Early rebleeding occurred in six patients and was managed successfully using endoscopy (n = 2), reembolization (n = 1), or surgery (n = 3). No major complications related to TAE occurred. Seven patients died within 30 days of TAE and three died later during the follow-up, but none of the deaths were due to rebleeding. No late bleeding recurrences were reported. These investigators concluded that selective TAE is safe and effective for controlling life-threatening bleeding from gastroduodenal ulcers, usually obviating the need for emergency surgery in critically ill patients, whose immediate survival depends on their underlying conditions.
Previous reports also evaluated the efficacy and safety of TAE. In a 6-year review of 40 consecutive patients with bleeding/rebleeding after endoscopic therapy and/or surgery for duodenal ulcer, superselective angiographic catheterization and coil embolization were performed by the same interventional radiologist. Lasting hemostasis was achieved in 26 of 40 patients (65%). Transfusion requirement was reduced from median 14 (range, 3–35) units of blood before TAE to two (range, 0–53) units after TAE. Ten patients died, half of them because of continuous bleeding. No adverse effects as a result of TAE were observed.
A recent retrospective review identified all patients admitted to Ullevål University Hospital with hematemesis and/or melena and endoscopically verified duodenal ulcer from June 2000 to 2005. The indication for TAE was endoscopically unmanageable bleeding/rebleeding or rebleeding after surgery. Technical success was defined as acute hemostasis. Clinical success was defined as technical success without rebleeding within 30 days. A total of 278 patients (mean age, 73 years) were included in the study. Primary endoscopic hemostasis failed in 13 patients (5%) and 53 patients (20%) experienced rebleeding. An attempt was made to treat 36 patients with TAE. Technical success in the TAE group was 92% and clinical success was 72%. In total, 10 patients underwent surgery, three because of rebleeding after TAE. The 30-day mortality was 10% for all patients, 19% in the TAE group, and 20% in the surgical group. High technical and clinical success was obtained with TAE in patients with bleeding duodenal ulcer after failure of endoscopic treatment in this cohort study.
A retrospective review of the outcome of TAE and surgery as salvage therapy of UGI bleeding after failed endoscopic treatment was recently performed in 658 patients referred for diagnostic/therapeutic emergency endoscopy and diagnosed with UGI bleeding (January 1998–December 2005). Of these 658 patients, 91 (14%) had repeat bleeding or continued to bleed. Forty of those 91 patients were treated with TAE and 51 were underwent surgery. Patients treated with TAE were older (mean age, 76 years; age range, 40–94 years) and had slightly more comorbidities compared with patients who underwent surgery (mean age, 71 years; age range, 45–89 years). The 30-day mortality rate in patients treated with TAE was 1 of 40 (3%) compared with 7 of 51 (14%) in patients who underwent surgery ( P <.07). Most repeat bleeding could be effectively treated with TAE, both in the surgical and TAE groups. The results of this study suggest that, after failure of therapeutic endoscopy for UGI bleeding, TAE should be the treatment of choice before surgery and that TAE can also be used to effectively control bleeding after failed surgery or TAE. There was a clear trend to lower 30-day mortality with the use of TAE instead of surgery.
The data from these cohort studies document that TAE is an effective and safe treatment in a significant proportion of patients with bleeding/rebleeding duodenal ulcers after therapeutic endoscopy and/or surgery and may serve as an alternative to surgery in high-risk patients ( Fig. 2 ).







