Despite improved understanding of peptic ulcer disease (PUD) pathogenesis, advances in diagnostic modalities, and the availability of modern pharmalogical, endoscopic and surgical treatments, gastroduodenal ulcer remains a major cause of morbidity and mortality worldwide. The predominant risk factors of this disorder remain Helicobacter pylori and ulcerogenic drugs. However, the proportion of idiopathic PUD is increasing worldwide often coinciding with the declining prevalence of H pylori infection. PUD heterogeneity worldwide is due to host genetic, bacterial and environmental factors. Variable ages in the acquisition of H pylori may influence the distribution of gastric versus duodenal ulcers as has the increasing use of aspirin and non-steroidal anti-inflammatory drugs (NSAIDs). Pharmacogenetics affects the magnitude of PUD risk induced by ulcerogenic drugs such as NSAIDs and also efficacy of proton pump inhibitors. Parietal cell mass and maximal acid output is higher in Western populations but it remains uncertain whether susceptibility to drug-induced PUD is influenced by race. H pylori antibiotic resistance also varies throughout the world. This review summarizes the similarities and differences in PUD aetiology, clinical features and treatment between the East and the West. In particular, we focus on the recent publications on the prevalence of H pylori , which is declining rapidly in various parts of the world resulting in overlapping prevalence rates in the East and the West.
Although the “East” and the “West” are loose terms based originally in differentiating ancient civilizations, their cultures, religions, and subsequent colonization to other parts of the world, this review differentiates the East and the West based on racial and ethnic differences, genetic ancestry, customs and lifestyles, and geography. These factors may have particular relevance in the development of peptic ulcer disease (PUD). In particular, emphasis is laid on reviewing the similarities and differences between the two based on the epidemiology, clinical manifestations, treatments, and complications of PUD.
A systematic review of the literature was performed. Publications were selected based on those that clearly distinguished populations descended from the Western civilization from their Eastern counterparts. The “West” was defined as Caucasian populations of Western and Central European ancestry. The “East” referred to populations in South, South-East, and Far-East Asia. Studies on populations from the Middle East, South and Central America, and Africa were excluded. A search of published literature was performed using PubMed from 1966 through December 2008 using the search terms “peptic ulcer,” “Asia(n),” and “Western/Caucasian.” In addition, in the evaluation of causative factors for PUD, the search terms “prevalence/epidemiology,” “ Helicobacter pylori ,” and “nonsteroidal anti-inflammatory drugs” were used. H pylori prevalence studies in specific disease states were excluded, because they represented highly selected populations. Only recent publications from the end of 2006 to 2008 were included to reflect the declining H pylori prevalence rates worldwide. The literature was reviewed for studies on PUD epidemiology, causes, clinical features, and management directly comparing Eastern and Western populations. Studies on neoplastic, ischemic, or infective causes of gastroduodenal ulceration, gastroesophageal reflux disease, and inflammatory bowel diseases were excluded. Relevant clinical and basic science publications were identified, and the related references were also obtained. Authors were contacted to provide further material if required. From all eligible articles, one reviewer (R.W.L.) scrutinized the abstracts to finally select the relevant publications.
Peptic ulcers
Epidemiology
Few publications directly compare PUD in the East with that in the West. The definition of PUD is mostly consistent in both the East and the West, although it is not explicitly expressed in most publications. The histopathologic description of PUD encompasses an epithelial defect, usually referring to the stomach or duodenum that extends down through the muscularis mucosae into the submucosa. The endoscopic definition usually requires the ulcerated surface breach to be obvious and with perceivable depth.
Accurate descriptive epidemiology of PUD is difficult to ascertain, making direct comparisons difficult between the East and the West. Most peptic ulcers are asymptomatic, and diagnosis relies on radiology or gastroscopy. The availability of these tests varies according to local health care systems and accessibility to these tests by patients. Reported incidence rates may be biased toward symptomatic presentations, development of overt clinical complications such as hemorrhage or perforation, and mortality. As such, there is an underestimate of the true disease prevalence. The true point prevalence of PUD can be obtained only through invasive diagnostic procedures, such as with gastroscopy, on the general population. There are, however, few such studies given the risks and discomfort associated with this investigation. A Swedish adult study that performed gastroscopies on random population samples found the prevalence of PUD to be 4.1% with equal proportions of GUs and DUs. The presence of PUD also correlated poorly with symptoms. A population-based endoscopy survey conducted in rural China found the PUD rate to be 9.3%. A total of 2423 individuals underwent gastroscopy. Of these, 137 had DUs (5.7%), 82 had non-malignant GUs (3.4%), and five had both DUs and GUs (0.2%). These population-based studies may be representative of the Western and Eastern populations respectively, but there is probably greater heterogeneity in different populations within these regions. Differences in the background risk factors, especially with the H pylori infection rate and use of ulcerogenic drugs, are the likely causes of heterogeneity of these results.
In the past, many Asian countries tended to have a higher DU-to-GU ratio. However, in many Asian countries there has been a gradual decline in this relationship. This most likely represents the declining prevalence of H pylori infection, which decreases DU risk and increasing use of aspirin and NSAIDs, which correspondingly increase the rate of GUs. A South Korean study of 895 patients with newly diagnosed PUD from Sep 2004 to Feb 2005 showed that the proportion of GU significantly increased from 44% to 48% with a corresponding decrease of DU from 45% to 39%. In a retrospective review of 15,341 endoscopies performed in the Philippines, the overall prevalence of PUD and H pylori infection have decreased over the past 7 years from 36% to 19%. The study also noted a significant increase in GUs presenting over time. In the pediatric population, upper gastrointestinal tract bleeding (UGIB) in Asia is more frequently caused by DUs. In the study by Guo and Zhan in China, DUs accounted for 54% of children with UGIB. Recent studies from Hong Kong found that DUs accounted for 75% of UGIB, with the majority being caused by H pylori . There is a male predisposition in presentation with UGIB in Asia, with a rate ranging from 1.9:1 to 2.6:1. Similarly, the endoscopic finding of PUD has decreased in Australia. A retrospective review from 1990 to 1998 showed the prevalence of PUD decreasing significantly from 22% to 13%. Despite the retrospective nature of these studies, there is agreement that PUD is decreasing in recent years in both the East and the West.
Historical Perspectives
PUD natural history has changed over the last 2 centuries. Data from the West show PUD to be rare before the 1800s. From the mid 1800s, GUs predominated over DUs. In the 1900s the prevalence of DUs increased to several times that of GUs. In the United States and Canada, hospitalization for PUD declined in the 1950s to 1970s, especially in males with PUD hemorrhage. Then there were increasing proportions of females and the elderly who presented with complicated PUD. In the United Kingdom, in a population study of 7590 incident PUD cases (DUs, 5564; GUs, 2026) from 1977 to 2001, presentation peaked in 1982 to 1986 and declined thereafter. There is a falling male preponderance, and GU patients tended to be older than DU patients. The mean age of both GU and DU patients increased over time. In the 1990s, there was a rise in PUD hemorrhage in the elderly, particularly from DU. GU perforations declined, but DU perforations increased among elderly men.
In an analysis of PUD mortality data from 1921 to 2004 from 6 European countries, there was a high correlation of data on PUD. The mortality rate of PUD increased among consecutive generations during the second half of the nineteenth century until shortly before the turn of the century and then decreased in all successive generations. The time trends of GU preceded those of DU by 10 to 30 years. There are fewer epidemiology studies from Asia. In Japan, the risk of PUD also increased in birth cohorts born before the twentieth century and declined in subsequent generations, similar to Western data. Declining PUD mortality occurred in both the East and the West but occurred later in the East. A decline in DU with corresponding increase in GU, similar to the changes in the West, has been noted in Korea.
Temporal Trend Changes in Peptic Ulcer Disease
The initial increase in PUD between 1850 and 1950 was thought to be due to a shift in the age of acquisition of H pylori . With the improvement in community hygiene, acquisition of infection tended to be later in life during adolescence. As opposed to acquisition of H pylori in early childhood, which leads to pangastritis, gastric atrophy, and reduced acidity, acquisition of H pylori later in life in a mature acid-secreting mucosa tends to confine the bacteria to the gastric antrum, where the bacteria inhibits D-cell function, further increasing gastric acid secretion. This increase in gastric acidity would increase PUD prevalence and in particular DUs. The decrease in PUD death rates among generations born during the twentieth century is usually attributed to the improving standards of hygiene in developed countries and receding infection by H pylori . The increase in aspirin and/or NSAID consumption and the introduction of potent antisecretory medications probably have not affected the long-term downward trends of ulcer mortality. The use of ulcerogenic drugs, however, has contributed to the increased proportion of the GU component of PUD in recent years. Overall, data from the East and West support a birth-cohort effect of PUD.
Risk factors
Gastric Physiology
Previous studies had found lower parietal cell mass in the Chinese compared with Caucasians. Parietal cell mass and maximal acid output after pentagastrin stimulation are higher in Caucasians than those in the Chinese. This difference was still present when the acid output was corrected for body weight with an acid output of 0.25 ± 0.11 mmol/h/kg for the Chinese and 0.30 ± 0.10 for Scots ( P <.01) as well as after controlling for sex and age. A higher H pylori prevalence in the Chinese, causing atrophic gastritis, may also contribute toward racial differences in gastric physiology.
Helicobacter pylori
H pylori infection plays an important part in the development of PUD. Because infection with the organism is typically subclinical, information on its prevalence is based mainly on serologic studies. Infection with H pylori in developing countries occurs at an earlier age than that in developed countries.
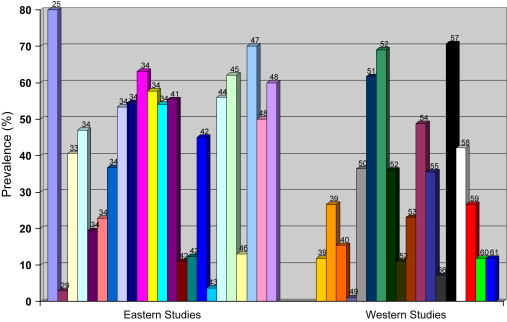
Given the geographic variability and recent changes in H pylori prevalence, a review was conducted on recent publications on H pylori prevalence in the East and West. From a total of 725 articles from a Pubmed search using the terms “ Helicobacter pylori ” and “prevalence” published in late 2006 to 2008, 12 relevant prevalence studies from Asia and 13 from the West were found. An additional 17 articles on H pylori prevalence in other parts of the world or studies performed in highly selected populations were excluded. The prevalence of H pylori from these studies is summarized in Table 1 .
| Authors and Reference | Population | Region and Sample Size | Age Range (y) | Diagnostic Test | Prevalence (%) |
|---|---|---|---|---|---|
| Asia | |||||
| Ahmed et al. | Hospital based | South India (500) | 30–79 | Gastric biopsy, 16S rRNA | 80.0 |
| Saragih et al. | Endoscopy based | Indonesia | — | Histology | 2.9 |
| Lee at al. | Endoscopy based | South Korea (8646) | >/=16 | RUT | 40.6 |
| Chen et al. | Population based | South China (1471) | Total | Serology | 47.0 |
| (180) | 1–4 | — | 19.4 | ||
| (105) | 5–9 | — | 22.9 | ||
| (185) | 10–19 | — | 36.8 | ||
| (253) | 20–29 | — | 53.4 | ||
| (196) | 30–39 | — | 54.6 | ||
| (204) | 40–49 | — | 63.2 | ||
| (163) | 50–59 | — | 57.7 | ||
| (185) | 60–69 | — | 54.1 | ||
| Fujimoto et al. | Population based | 3 areas, Japan (3819) | 20–70+ | Serology | 55.4 |
| Lin et al. | Community based | Central Taiwan healthy school children (1625) | 9–12 | Serology | 11.0 |
| Healthy school children (325) | 13–15 | — | 12.3 | ||
| Teacher (253) | >/=25 | — | 45.1 | ||
| Okuda et al. | Birth cohort | Japan (108) | 2 | Stool antigen | 3.7 |
| Yim et al. | Population based | South Korea (15,916) | 16 | Serology | 56.0 |
| Shi et al. | Population based | Jiangsu province, China (1371) | 5–100 | UBT, serology | 62.1 |
| Tam et al. | Population based | Hong Kong (2480) | 6–19 | UBT | 13.1 |
| Yanaoka et al. | Population based, males | Western Japan (5,209) | 40–60 | Serology | 70.1 |
| Bhuiyan et al. | Birth cohort study | Bangladesh (238) | 0–2 | Fecal antigen | 50 |
| Serology | 60 | ||||
| Caucasian | |||||
| Moujaber et al. | Laboratory blood samples | Australians (2413) | 1–59 | Serology | 15.4 |
| Gruber et al. | Hospital based | Swiss-born (101) | 18–85 | UBT | 11.9 |
| Non-Swiss-born (252) | — | — | 26.6 | ||
| Mourad-Baars et al. | Population based | Zuid-Holland, The Netherlands (1258) | 2–4 | Serology | 1.2% |
| Dinic et al. | School based | Nis, Serbia (283) | 7–18 | Serology | 36.4 |
| Boyanova et al. | Endoscopy based | Bulgaria (658) | 1–17 | RUT, histology, culture | 61.7 |
| Thjodleifsson et al. | Population based | Tartu, Estonia (240) | 25–50 | Serology | 69 |
| Reykjavik, Iceland (447) | — | — | 36 | ||
| Uppsala, Sweden (359) | — | — | 11 | ||
| Naja et al. | Population based | Ontario, Canada (1306) | 50–80 | Serology | 23.1 |
| Weck et al. | Population based | Saarland, Germany (9444) | 50–74 | Serology | 48.8 |
| Sterzl et al. | Population based | Czech Republic (1621) | Mean age, 27 | Serology | 35.6 |
| Segal et al. | Endoscopy based | Canadians (204) | 5–18 | Histology, RUT, UBT, HpSA | 7.1 |
| Monno et al. | Healthy volunteers | Albanians (1088) | 16–64 | Serology | 70.7 |
| Rejchrt et al. | Hospital-based | Czech Republic (1810) | 5–100 | UBT | 42.1 |
| Gruber et al. | Hospital-based | — | 18–85 | UBT | — |
| Non-Swiss-born (252) | — | — | 26.6 | ||
| Swiss-born (101) | — | — | 11.9 | ||
The prevalence of H pylori infection varies widely both between and within populations based on geography, age, race, ethnicity, and socioeconomic status (SES). Overall, inadequate sanitation practices, low social class, crowded or high-density living conditions, and inadequate nutritional status are associated with a higher prevalence of infection. Based on this, the rate of acquisition is generally higher in developing countries than that in industrialized countries.
Helicobacter pylori in the East
Traditionally, Asian countries demonstrated overall higher prevalence rates of H pylori compared with those in Western countries. In a study of 500 hospital patients from South India, the overall prevalence of H pylori was 80%. The prevalence rate varied according to the source of drinking water (prevalence of 92% when water came from wells compared with 74.8% from taps), socioeconomic status (SES; 86.1% with lower SES compared with 70% from higher SES), and crowded living conditions (high density, 83.7%; medium density, 76.6%; low density, 71.3%; all statistically significant). The greatest contribution toward H pylori prevalence was the clean water index (CWI), with a prevalence rate of 88.2% in those with access to low CWI and as low as 33.3% from those with high CWI. The CWI reflected whether water is stored and reused, frequency of bathing, and whether water is boiled before drinking. These practices are highly variable within Asia and even within communities. Cultural practices and ethnicity may play additional roles in the higher rates of H pylori infection in Asia and other developing countries. In rural areas of China, the rate of mother-to-child transmission of H pylori may increase due to the traditional practice of close maternal contact with their children, including sharing the same bed, eating from the same bowls, using the same chopsticks, and even feeding infants by prechewing their food. Cultural practices and local environmental conditions are likely to perpetuate the high rates of infection in Asia.
There are ethnic differences in the pattern of H pylori gastritis. Koreans and Japanese are more likely to have antral involvement compared with Americans and more likely to develop interstitial neutrophilic infiltration, intestinal metaplasia, and atrophy. In patients with DUs, H pylori infection in the stomach usually results in antral-predominant gastritis, with a lower likelihood of corpus gastritis and intestinal metaplasia. Geographically, however, there is a difference in the risk of intestinal metaplasia in patients with DUs, with a significantly higher rate of intestinal metaplasia in Korea (38%) compared with that in Caucasian populations (10%, P = .018), indicating variability in the effects of H pylori .
Within countries, there are also differences in H pylori prevalence rates between races. In Malaysia, differential prevalence rates of H pylori are noted among the Malay, Chinese, and Indian populations, with prevalence rates lowest in Malays (11.9%–29.2%), intermediate in the Chinese (26.7%–57.5%) and highest in Indians (49.4%–52.3%). In contrast to other parts of Asia, Indonesia has a disproportionately low prevalence rate of H pylori infection. Over a 14-year period from 1998 to 2005, the prevalence of H pylori decreased from 12.8% to 2.9%. Similarly low H pylori prevalence rates of 4% to –8% have also been described in the Malay population in Malaysia. These prevalence rates are unexpected from a developing Asian country, and it is hypothesized that such populations with unexpectedly low prevalence or absence of H pylori may have descended from uninfected founding populations. Alternatively, there may be as yet unknown host mechanisms that resist transmission and acquisition of the organism.
With the economic development of many parts of Asia, increasing prosperity and industrialization, and improving hygienic practices, several longitudinal studies from Asia have demonstrated declining H pylori seroprevalence. Serum from the National Institute of Infectious Diseases in Tokyo revealed a declining H pylori seroprevalence from 73% in 1974 to 55% in 1984 and to 39% in 1994. In South Korea, the infection rate was 50.0% in 1997 but declined down to 40.6% in 2005 ( P <.001). In Guangzhou, southern China, the age-standardized seroprevalence of H pylori decreased from 62.5% in 1993 to 49.3% in 2003. A decrease in H pylori prevalence in children represents a true decline in H pylori , especially in the coming years given the age-cohort effect in the acquisition of infection. In the pediatric population, many parts of China now report decrease in H pylori prevalence. In Linqu County of northeast China, the prevalence rate increased from 50% at ages 3 to 4 years to 85% at ages 9 to 10 years before falling to 67% at ages 11 to 12 years. Previously, the high prevalence of H pylori at an early age was linked to the high rate of chronic atrophic gastritis and progression to gastric cancer in adults, especially in this region. In Guangzhou, the prevalence of children with H pylori decreased from 30.8% to 19.4% during 10 years. Hong Kong, in southern China, is characterized by rapid Westernization and industrialization. The H pylori prevalence has remained stable at 25% over time. In this region, there is a high standard of hygiene, but overcrowding and familial clustering are thought to play important roles perpetuating H pylori transmission. H pylori either alone or in combination with ulcerogenic drugs is still a major cause of UGIB and accounted for 73.5% to 90.2% of bleeding in the pediatric population.
Helicobacter pylori in the West
In contrast, Western countries generally have a lower rate of H pylori infection in comparison with Asia. Based on 13C urea breath test, the rate of H pylori infection in the Switzerland-born population was 11.9%, which was significantly lower than 26.6% ( P = .003) for those residing in Switzerland but born outside of the country. In Australia, the overall seroprevalence of H pylori infection was 15.1% in 2002 with no difference between genders. Seropositivity rates increased progressively with age from 4% in 1- to 4-year-olds to 23.3% in 50- to 59-year-olds.
In general, there has been a decline in the rate of H pylori infection in many parts of the world. The risk of infection remains the same—overcrowding, unclean water sources, and possibly cultural behaviors that increase the rate of transmission between mother and babies maintain the higher prevalence rates in Asia. Asian countries that have become more Westernized have demonstrated reduction in the prevalence of H pylori infection, especially in the pediatric population. In the peak prevalence age group of 50- to 59-year-olds, however, the infection rate continues to be more than 50%. This group of patients therefore are at risk of developing PUD and other complications of H pylori infection. Fig. 1 illustrates the varied and overlapping H pylori prevalence rates in the East and the West and their references.
Risk factors
Gastric Physiology
Previous studies had found lower parietal cell mass in the Chinese compared with Caucasians. Parietal cell mass and maximal acid output after pentagastrin stimulation are higher in Caucasians than those in the Chinese. This difference was still present when the acid output was corrected for body weight with an acid output of 0.25 ± 0.11 mmol/h/kg for the Chinese and 0.30 ± 0.10 for Scots ( P <.01) as well as after controlling for sex and age. A higher H pylori prevalence in the Chinese, causing atrophic gastritis, may also contribute toward racial differences in gastric physiology.
Helicobacter pylori
H pylori infection plays an important part in the development of PUD. Because infection with the organism is typically subclinical, information on its prevalence is based mainly on serologic studies. Infection with H pylori in developing countries occurs at an earlier age than that in developed countries.
Given the geographic variability and recent changes in H pylori prevalence, a review was conducted on recent publications on H pylori prevalence in the East and West. From a total of 725 articles from a Pubmed search using the terms “ Helicobacter pylori ” and “prevalence” published in late 2006 to 2008, 12 relevant prevalence studies from Asia and 13 from the West were found. An additional 17 articles on H pylori prevalence in other parts of the world or studies performed in highly selected populations were excluded. The prevalence of H pylori from these studies is summarized in Table 1 .
| Authors and Reference | Population | Region and Sample Size | Age Range (y) | Diagnostic Test | Prevalence (%) |
|---|---|---|---|---|---|
| Asia | |||||
| Ahmed et al. | Hospital based | South India (500) | 30–79 | Gastric biopsy, 16S rRNA | 80.0 |
| Saragih et al. | Endoscopy based | Indonesia | — | Histology | 2.9 |
| Lee at al. | Endoscopy based | South Korea (8646) | >/=16 | RUT | 40.6 |
| Chen et al. | Population based | South China (1471) | Total | Serology | 47.0 |
| (180) | 1–4 | — | 19.4 | ||
| (105) | 5–9 | — | 22.9 | ||
| (185) | 10–19 | — | 36.8 | ||
| (253) | 20–29 | — | 53.4 | ||
| (196) | 30–39 | — | 54.6 | ||
| (204) | 40–49 | — | 63.2 | ||
| (163) | 50–59 | — | 57.7 | ||
| (185) | 60–69 | — | 54.1 | ||
| Fujimoto et al. | Population based | 3 areas, Japan (3819) | 20–70+ | Serology | 55.4 |
| Lin et al. | Community based | Central Taiwan healthy school children (1625) | 9–12 | Serology | 11.0 |
| Healthy school children (325) | 13–15 | — | 12.3 | ||
| Teacher (253) | >/=25 | — | 45.1 | ||
| Okuda et al. | Birth cohort | Japan (108) | 2 | Stool antigen | 3.7 |
| Yim et al. | Population based | South Korea (15,916) | 16 | Serology | 56.0 |
| Shi et al. | Population based | Jiangsu province, China (1371) | 5–100 | UBT, serology | 62.1 |
| Tam et al. | Population based | Hong Kong (2480) | 6–19 | UBT | 13.1 |
| Yanaoka et al. | Population based, males | Western Japan (5,209) | 40–60 | Serology | 70.1 |
| Bhuiyan et al. | Birth cohort study | Bangladesh (238) | 0–2 | Fecal antigen | 50 |
| Serology | 60 | ||||
| Caucasian | |||||
| Moujaber et al. | Laboratory blood samples | Australians (2413) | 1–59 | Serology | 15.4 |
| Gruber et al. | Hospital based | Swiss-born (101) | 18–85 | UBT | 11.9 |
| Non-Swiss-born (252) | — | — | 26.6 | ||
| Mourad-Baars et al. | Population based | Zuid-Holland, The Netherlands (1258) | 2–4 | Serology | 1.2% |
| Dinic et al. | School based | Nis, Serbia (283) | 7–18 | Serology | 36.4 |
| Boyanova et al. | Endoscopy based | Bulgaria (658) | 1–17 | RUT, histology, culture | 61.7 |
| Thjodleifsson et al. | Population based | Tartu, Estonia (240) | 25–50 | Serology | 69 |
| Reykjavik, Iceland (447) | — | — | 36 | ||
| Uppsala, Sweden (359) | — | — | 11 | ||
| Naja et al. | Population based | Ontario, Canada (1306) | 50–80 | Serology | 23.1 |
| Weck et al. | Population based | Saarland, Germany (9444) | 50–74 | Serology | 48.8 |
| Sterzl et al. | Population based | Czech Republic (1621) | Mean age, 27 | Serology | 35.6 |
| Segal et al. | Endoscopy based | Canadians (204) | 5–18 | Histology, RUT, UBT, HpSA | 7.1 |
| Monno et al. | Healthy volunteers | Albanians (1088) | 16–64 | Serology | 70.7 |
| Rejchrt et al. | Hospital-based | Czech Republic (1810) | 5–100 | UBT | 42.1 |
| Gruber et al. | Hospital-based | — | 18–85 | UBT | — |
| Non-Swiss-born (252) | — | — | 26.6 | ||
| Swiss-born (101) | — | — | 11.9 | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





