and Andrea Bischoff1
(1)
Pediatric Surgery, Colorectal Center for Children Cincinnati Children’s Hospital, Cincinnati, OH, USA
Electronic supplementary material
Supplementary material is available in the online version of this chapter at 10.1007/978-3-319-14989-9_10.
10.1 Introduction
This malformation is defined as a defect in which the rectum is abnormally communicating with the middle portion of the posterior urethra, also known as the prostatic urethra, and there is no anal opening (Fig. 10.1). This is the second most common anorectal malformation defect in males seen by us [1]. Until the moment of writing this manuscript, our experience included 227 patients operated by us with prostatic fistula: 193 of them were primary and 34 were reoperations. Rectoprostatic fistula represents a defect considered intermediate in terms of complexity between a rectourethral bulbar fistula and a recto-bladder neck fistula. Many authors [2, 3] do not make a distinction between rectourethral bulbar fistula and rectourethral prostatic fistula, but rather consider both groups together and use a single generic name, which is “rectourethral fistula.” We believe that it is important to differentiate these two groups (prostatic and bulbar) because they have different therapeutic implications and different functional prognoses. In addition, the frequency of association of other defects is significantly different between these three groups (bulbar, prostatic, and bladder neck).
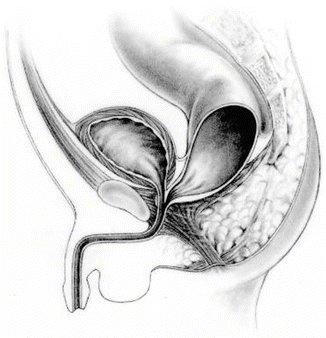

Fig. 10.1
Diagram showing a prostatic fistula
As we previously mentioned, Mother Nature does not respect the classifications that we create in order to communicate between ourselves. Thus, a rectourethral prostatic fistula sometimes is located a little close to the bulbar urethra or sometimes is located close to the bladder neck.
10.2 Associated Defects
The general frequency of association of other defects is higher than in cases of rectourethral bulbar cases. Absent kidney occurs in 10 % of cases, hydronephrosis in 6 % of cases, vesicoureteral reflux in 26 % of cases, and hypospadias in 7.3 % of cases. Undescended testicles were present in 8 % of these cases. Ten percent of the patients had a bifid scrotum. As can be seen, the high frequency of associated urologic defects puts this malformation into a category of serious defect. The average AP sacral ratio for a patient with rectoprostatic fistula is 0.55 for patients with voluntary bowel movements and 0.47 for those who are fecally incontinent. The lateral average ratio is 0.64 for continent patients and 0.58 for incontinent patients, which is significantly lower than the sacral ratio of patients with rectourethral bulbar fistula and significantly higher than the ratio for cases with for recto-bladder neck fistulas. Twenty-two percent of rectoprostatic fistula patients suffer from tethered cord which, again, is a higher incidence than in cases of rectourethral bulbar fistula. Hemivertebrae occur in 8 % of cases, and they occur mainly in the lumbar spine. Esophageal atresia occurs in 14 % of cases and duodenal atresia in 2 % of cases. Patent ductus arteriosus occurs in 7 % of cases, but only one fourth of them require some sort of intervention due to hemodynamic problems. Atrial septum defects occur in 8 % of cases but did not require any type of treatment. Ventricular septum defects occur in 6 % of cases, but only one third of them required therapeutic intervention. Tetralogy of Fallot occurred in 2 % of cases.
As previously suggested, the common wall located between the rectum and the urethra above the fistula site is shorter in cases of rectoprostatic fistula when compared to those of rectourethral bulbar fistula (Fig. 10.1). This fact makes the separation of the rectum from the urethra technically easier. Yet, once the rectum has been separated from the urinary tract, the mobilization required in order to pull the rectum down is a more complex and technically demanding maneuver.
Some of the rectoprostatic fistulas are located close the bladder neck but not quite into the bladder and therefore can be approached by both a posterior sagittal incision and laparoscopically through the abdomen. This particular type of defect represents a matter of controversy in terms of which approach is better. We believe that a surgeon that has experience with the posterior sagittal approach can easily and safely find the rectum posterior sagittally, separate it from the urinary tract, and mobilize the rectum down safely, provided the colostomy is well located (not too distal). We also believe that a well-trained laparoscopic surgeon can relatively easily separate the rectum from the urinary tract via laparoscopy. In other words, we believe that to decide how to approach these patients is something that should be done based on the specific circumstances of the surgeon and the patient. Some of the serious catastrophes and failed attempted repairs that we have seen happening at other institutions precisely occurred in these types of high prostatic fistulas. In retrospect, the surgeon either did not have a good distal colostogram (Animation 10.1) or simply operated on the patient without a distal colostogram, entered posterior sagittally looking for a rectum that was located much higher than what he thought, could not find the rectum, but rather found structures that he was not looking for, such as seminal vesicles, vas deferens, urethra, or during the search, damaged important nerves of the urogenital tract, resulting in neurogenic bladder, a complication considered totally preventable [4].
The perineum of patients with prostatic fistula may show signs of what we call bad prognosis. The midline groove may not be so prominent, and the anal dimple may not be represented by a real fossette but rather by a group of fibers in the midline raphe (Fig. 10.2).
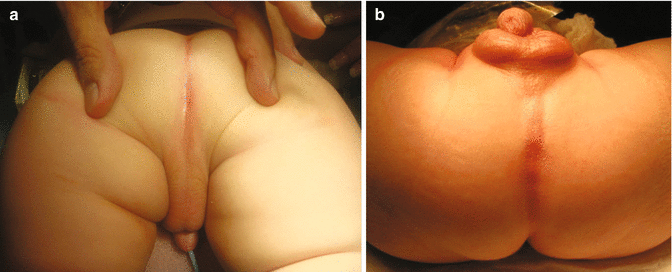

Fig. 10.2
Photograph showing the perineum of two patients with prostatic fistula. (a) prone position. (b) supine position
We believe that all patients born with rectoprostatic fistula benefit from a diverting colostomy at birth and the malformation must be repaired in a second operation. One of the main arguments in favor of this approach is the fact that the colostomy, in addition to decompressing the gastrointestinal tract and saving the baby’s life, allows us to perform a good high-pressure distal colostogram, which is the only and best way to provide information about the precise location of the rectum and the fistula (Fig. 10.3). It is this study that allows us to follow a specific strategy during the repair of this malformation and to avoid catastrophic complications.
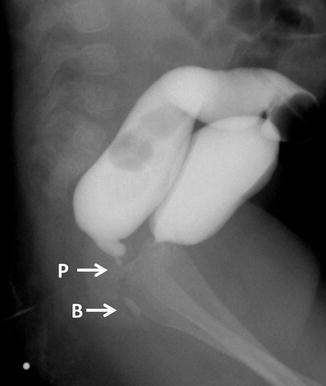

Fig. 10.3
Colostogram (BU and PR) comparing the images of a bulbar fistula with a prostatic. P = Prostatic, B = Bulbar
10.3 Surgical Repair
Two to four weeks after the colostomy has been opened, provided the patient is growing and developing normally, the main repair can be performed. It is not an urgent procedure; if the surgeon is not familiarized with the anatomy of little babies, he can wait until the baby is bigger or reaches the size that the surgeon is accustomed to operate on.
10.4 Posterior Sagittal Anorectoplasty (Animation 10.2)
It is our routine to perform a cystoscopy in all of these patients, and that is how we have been learning important anatomic details of the posterior urethra. We have found that there is a spectrum of defects in the posterior urethra, including the presence of ectopic ureters and abnormalities in the verumontanum. Once we finish the cystoscopy, a no. 8 Foley catheter is introduced through the urethra and into the bladder. In general, the Foley catheter is passed without difficulty; it does not go into the rectum. Occasionally, however, there is a kink of the urethra at the location of the fistula, which may interfere with the passing of the Foley catheter. Sometimes, in addition to the kink, there is a real congenital stenosis. The posterior sagittal approach is ideal to repair abnormalities of the posterior urethra at the same time than the repair of the anorectal malformation.
The higher the location of the fistula, the more frequently we may see ectopic ureters. When these abnormal ureters are ectopically connected to the posterior urethra, they must be dealt with, during the posterior sagittal approach.
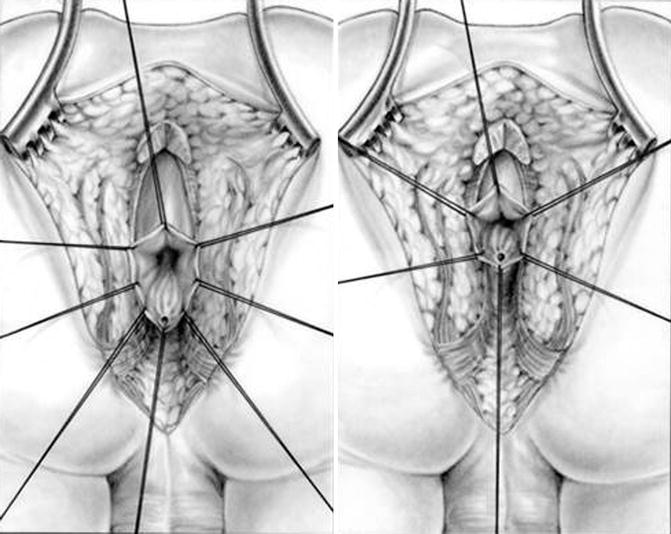
The patient is positioned prone as previously described for the posterior sagittal approach. The posterior sagittal incision runs from the middle portion of the sacrum to the anal dimple. We put special emphasis in making the incision exactly midline using the electrical stimulator to try to leave equal amounts of muscle in both sides of midline. We go through the skin, subcutaneous tissue, parasagittal fibers, ischiorectal fossa, and levator mechanism (see Chap. 9, Sect. 9.4). Once we divide the levator mechanism, we have to keep in mind the image of the distal colostogram to determine where to exactly look for the rectum. Figure 10.4 shows a diagram of rectobulbar and rectoprostatic fistula. In general, in patients with rectoprostatic fistula, the surgeon must look for the rectum immediately below the coccyx. In those particular cases where the rectum seems to be located a little higher, close to the bladder neck, we have to look for the rectum above the coccyx. This is extremely important because the possibility of producing extra damage to the urogenital tract increases in cases of rectoprostatic fistula with a high rectum. The rectum in patients with rectoprostatic fistula is found to be much smaller than in cases with bulbar fistula. Unfortunately, the appearance of the white fascia after we have divided the levator muscle does not allow us to determine or predict where the rectum is going to be found. The distal colostogram is the main guideline that we should follow. Two silk stitches are placed as high as possible on the posterior aspect of what we think is the rectum in front of the coccyx or above the coccyx assuming that we are holding on the rectum (Fig. 10.5). Using traction on these silk sutures, we can divide the white fascia that covers the rectum, as well as the perirectal fat, bands, and vessels located deeper than the white fascia. By doing this, we notice that the rectal wall gives up, and we can mobilize it lower (Fig. 10.6). We continue the dissection, staying as close as possible to the bowel wall, dividing bands and vessels until we feel safe that we are actually dealing with the rectum. At that point, we make an incision on the posterior rectal wall in between the two stitches to find the rectal lumen (Fig. 10.7). The incision in the posterior rectal wall is extended caudally, placing silk sutures on the edges of the rectum until we find the fistula site, and the last 5-0 silk stitch is placed taking the lower edge of the fistula site (Fig. 10.8). Multiple 6-0 silk stitches are placed taking the mucosa of the anterior rectal wall in the upper hemi-circumference of the fistula. Those multiple stitches are included into a single clamp to apply uniform traction to facilitate the separation of the rectum from the urethra (Fig. 10.9). Needle-tip cautery is used to make an incision in the rectal mucosa between the multiple 5-0 silk stitches and the fistula site. This incision is barely 1 mm deep (see Chap. 9). At that point, we put together into a single mosquito clamp the silk stitches that were previously placed on one of the rectal edges; by applying uniform traction on the mosquito, we can clearly see the white fascia and the extrinsic blood supply of the rectum. The white fascia, fat, and vessels are resected, exposing a clean bowel wall which is the plane of dissection of the rectum. The same steps are repeated on the opposite side (see Chap. 9). With both lateral rectal walls clean, the dissection between the rectum and the urinary tract is started, having as a reference the lateral plane previously established. Fortunately, as we said before, the common wall between the rectum and urethra in cases of rectoprostatic fistulas is relatively short, usually about 5 mm in length. Very soon, we find that the rectum and urinary tract are completely separated structures (Fig. 10.10). At that point, a circumferential dissection is performed, aimed to gain length of the rectum. For that, we put all of the silk stitches that we originally placed in the edges of the rectal wall and those that we placed in the rectal mucosa into a single clamp, again, to apply uniform traction. Small malleable retractors are used, in order to identify the bands and vessels that hold the rectum up in the pelvis. The dissection is performed in a systematic circumferential manner dividing those bands. Dividing bands and vessels allows gaining length, which allows us to see new bands and vessels previously unrecognized. Sometimes, all what we can see is a groove, which represents a tense band. We grab those vessels, separate them from the bowel wall, and burn them, putting special emphasis on not burning the bowel wall. Very soon, the peritoneal reflection is identified and opened, which allows mobilizing the rectum even more. The dissection continues until the rectum is mobilized enough to be anastomosed to the anal dimple with no tension (Fig. 10.11).