and Andrea Bischoff1
(1)
Pediatric Surgery, Colorectal Center for Children Cincinnati Children’s Hospital, Cincinnati, OH, USA
8.1 Definition, Frequency, and Prognosis
Perineal fistula is an anal malformation in which the anal opening is located anterior to the center of the sphincter. In females, the anal opening is located somewhere between the location of the normal sphincter and the female genitalia in the area known as the perineum or perineal body. The anterior mislocation of the orifice could be minimal (a few millimeters) (Fig. 8.1) or severe, becoming borderline with a malformation called vestibular fistula (Fig. 8.2). When the anal orifice is located at the junction of both labia majora, the malformation sometimes receives the French name “fourchette malformation” (Fig. 8.3), which is considered a defect intermediate between the vestibular and perineal area. Yet, most anorectal malformations in females can be clearly differentiated between perineal and vestibular. This is, perhaps, the anorectal defect subjected to more controversies, both in semantics and treatments. In female patients, some surgeons use different terms to refer to this condition, including “ectopic anus” and “anterior displacement of the anus” [1–13]. We prefer the term perineal fistula for the following reasons:
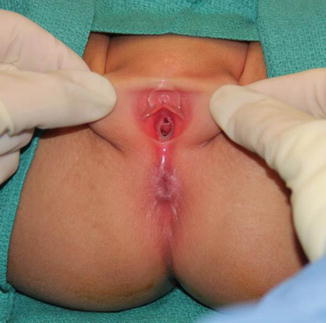
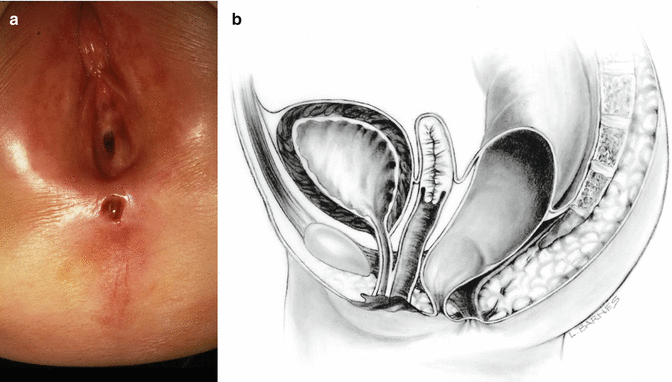
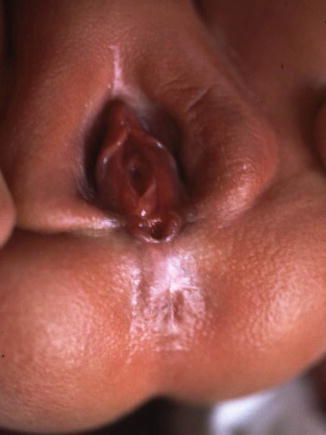

Fig. 8.1
Picture of a perineal fistula with minimal anterior mislocation of the opening

Fig. 8.2
Perineal fistula with a significant anterior mislocation of the anal opening. (a) Picture. (b) Diagram

Fig. 8.3
“Fourchette” type of fistula
The anal opening is most frequently strictured or stenotic.
There is no anal canal.
The orifice is not surrounded 360° by a sphincter mechanism.
These facts make us believe that this is not a real anus. For similar reasons, we believe that in order to call a malformation “anterior anus,” it would be necessary for the patient to have a non-stenotic orifice, with normal anal canal, and surrounded 360° by a sphincter mechanism (as electrically demonstrated). If we accept this, as the definition of an anterior anus, then we must say that we have never seen that specific type of defect. The fact that we have not seen such defect does not mean that it does not exist, but it certainly means that it must be extremely rare. In addition, we believe that in the event of seeing a case suffering from such condition, we would be very hesitant to operate.
Perineal fistula is one of the most common anorectal defects. Our series includes 90 males and 84 females, occupying the second place in frequency in males after the rectourethral fistulas (bulbar and prostatic) together and the third place in females after vestibular fistula and cloacas. However, we have reasons to believe that the perineal fistula is perhaps one of the two most frequent defects in females. Most likely, the majority of perineal fistula cases are not referred to us, probably because they are considered easy to repair. Ours is a referral center, and we receive mainly complex and (or) complicated patients from other institutions. That may be the reason to explain the elevated number of cloacas that we have, as well as the rather low number of cases with perineal fistula. We conclude that the relative frequency of presentation of these malformations in our series is not representative of the frequency in the general population.
Perineal fistulas are the most benign of all anorectal defects in terms of functional prognosis for bowel control. In fact, in our experience, 100 % of the patients operated by us have bowel control provided the sacrum is normal. The majority of patients have a good sacrum, but there are exceptions, as we will be discussing below.
The chances for these patients to have bowel control are 100 % provided they receive a good operation. Ironically, the incidence of constipation in these patients is the highest of the entire spectrum of anorectal malformations!! In fact, we observed that the higher the malformation, the poorer the prognosis for bowel control, but the better the prognosis for constipation, or the other way around, the lower and more benign the malformation, the highest chances of bowel control, but also the highest possibilities of suffering severe constipation. The reason for this is unknown. Interestingly, higher malformations require much more perirectal dissection in order to bring the rectum down to the perineum. This dissection means that we divide the vessels and the nerves that surround the rectum in order to mobilize it down. In other words, higher rectums require more denervation, yet they have less constipation!! Patients with perineal fistulas require a minimal degree of mobilization and therefore denervation, and yet they have the worst degree of constipation.
Another important characteristic of this particular defect is that there is a group of patients with perineal fistulas that run in families. It is well documented in the literature that about 1 % of all patients with anorectal malformations have a sibling with an anorectal malformation [14]. In other words, traditionally, when the parents ask about the risk of having a second child with an anorectal malformation, the standard answer from geneticists, pediatricians, and us is 1 %. Yet, a deeper and more thoughtful analysis of the question reveals that, actually, we have never seen a family with two cloacas. In contrast, we have several families that have two or more siblings with perineal fistula frequently associated to a presacral mass and sacral defect. The familiar characteristic of this condition has been published many times [15–31]. Some other members of the family may have the sacral defect and the presacral mass and not the perineal fistula. For this reason, in a patient with perineal fistula, it is mandatory to document the integrity of the sacrum with an AP x-ray film of the sacrum to rule out a sacral defect because of the high incidence of sacral defects and presacral masses associated to perineal fistulas. Once we document a case of perineal fistula with a presacral mass, we have to screen the other members of the family for the same defect. This is extremely important because we have seen patients that were diagnosed as having a perineal fistula received an operation focused only in the anal defect, and the patients were left with a presacral mass and sometimes with very negative consequences. In addition, having diagnosed a sacral defect with a presacral mass changes completely the functional prognosis for these patients. This, we believe, it is extremely important to diagnose, to discuss with the parents, and to adjust their expectations in terms of future functional prognosis. In addition, as will be described later, this complex triad requires a specific therapeutic strategy.
The association of anorectal malformation, sacral defect, and presacral mass is well known [32–61] and is frequently called Currarino triad [38].
Unfortunately, there is a group of patients who were born with perineal fistula, as previously mentioned, a defect with an excellent functional prognosis, and yet, they end up suffering from fecal incontinence after several therapeutic misadventures and catastrophic events. Those are totally preventable problems consecutive to the lack of knowledge of the management of these patients.
8.2 Associated Defects
Following the same pattern already observed in the entire spectrum of anorectal malformations, perineal fistulas have the lowest incidence of urologic-associated defects or functional disorders. According to our database, 18 % of female patients and 27 % male patients with perineal fistulas suffer from urologic problems. Absent kidney occurs in 2–4 % of the cases, vesicoureteral reflux in 3–5 %, hypospadias in 1 %, ectopic ureters in less than 1 %, vaginal septum in 2 %, hyposadias in 1 %, undescended testicle in 2 %, bifid scrotum in 8 %, and hydronephrosis in 6 %.
Vertebral anomalies also are less common in these defects as compared to the others. Hemivertebra occurs in less than 2–4 % of the cases, and sacral defects are very unusual. The sacra in these patients are usually normal except in those patients who are born with presacral mass and hemisacrum.
Patent ductus arteriosus occurs in less than 3 %, atrial septal defect in 2 %, ventricular septal defect in 1 %, tetralogy of Fallot in 1 %, and esophageal atresia in 1 %.
Tethered cord is present in 29 % of male patients and 13 % of female patients. This relatively high frequency of tethered cord is explained by the fact that patients with perineal fistula have a high frequency of association with sacral defects and presacral masses.
8.3 Diagnosis
8.3.1 Female Patients
The diagnosis of a perineal fistula in a female patient is a straightforward one. It is enough to see the perineum of the baby to understand that the anal orifice is abnormally located anterior to the center of the sphincter (Figs. 8.1 and 8.2). As previously mentioned, the orifice may be located very close to the female genitalia or very close to the center of the sphincter, and so the degree of mislocation varies from patient to patient. There is also a specific defect called by Stephens [62] “perineal groove” which is a strip of mucosa that runs between the female genitalia and the anus. This defect is frequently associated to perineal fistulas (Fig. 8.4). When this is left untouched, most of the times, that strip of mucosa suffers from metaplasia and changes into something that looks very much like skin. Yet, we have seen patients of 5–7 years old in which that mucosa continued producing some degree of wetness, which prompted us to operate. The operation of the perineal groove requires only removal of a very thin layer of mucosa and suturing together in the midline the skin of the perineal body. This operation is usually done in conjunction with the mobilization of the anal opening back to the center of the sphincter.
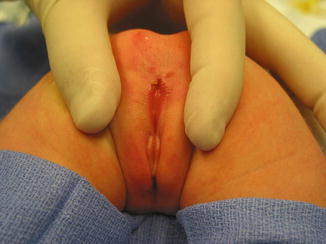

Fig. 8.4
Picture of a perineal groove
Sometimes, the anus looks like it is surrounded by a normal sphincter mechanism. Yet, with an electrical stimulator, under anesthesia, it is extremely easy to demonstrate that it is just an optical illusion. The bulk of the sphincter mechanism is located behind the anal opening and extends like a horseshoe on both sides of the anus, but there is no sphincter whatsoever between the female genitalia and the anal opening.
8.3.2 Male Patients
The diagnosis in the male patient may be a little bit trickier. The baby may have a very obvious orifice in the perineum through which one can see meconium coming out. In such case, the diagnosis is easy (Fig. 8.5). The fistula is always located anterior to the center of the sphincter. We have never seen a case in which the anal opening is located posterior to the center of the sphincter. The orifice, however, could vary in size from patient to patient being always stenotic. Sometimes there is a tiny, almost invisible orifice (Fig. 8.6). The fact that it is a small orifice usually delays the passing of meconium, which is another reason why we believe that in the management of the newborn baby with anorectal malformation, decisions concerning the opening of a colostomy or anorectal repair should not be taken before 24 h. In the first 24 h, it is very unlikely for the patient to pass meconium through a tiny orifice. When the pediatric surgeon is called to see a baby with imperforate anus, it is extremely important to do a meticulous inspection of the perineum. One should not hesitate to use magnifying glasses if necessary to look carefully for an orifice that sometimes is inconspicuous. Figures 8.7, 8.8, 8.9, and 8.10 show different appearances of the same malformation (perineal fistula).
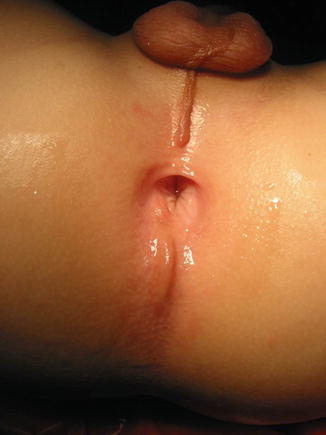
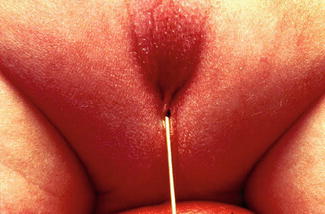
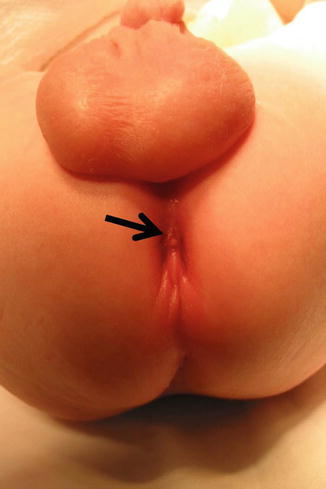
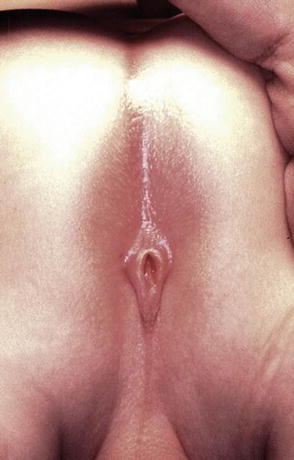
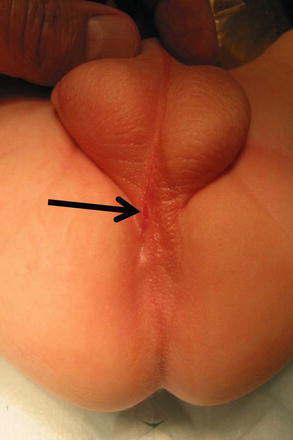
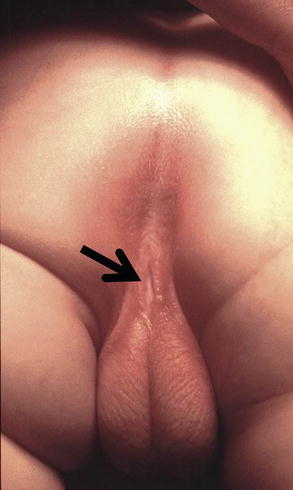

Fig. 8.5
Perineal fistula in a male. The anal orifice is very obvious

Fig. 8.6
Very small perineal fistula

Fig. 8.7
Arrow shows fistula opening

Fig. 8.8
The discoloration of the skin, posterior to the fistula opening represents the location of the sphincter

Fig. 8.9
Arrow shows fistula opening a little out of the midline

Fig. 8.10
Arrow shows fistula opening at the base of the scrotum
We intentionally eliminated confusing terms used in the past to refer to this defect. These terms include: “anal membrane,” “membranous stenosis,” “covered anus,” “anocutaneous fistula,” and “translevator defect” [63–68].
The orifice may go undetected. This may have important therapeutic implications. We have seen 17 patients born with this defect in which the surgeon was unaware of the presence of a perineal orifice, believed that the patient had a “high anorectal malformation” opened a non-indicated colostomy. Subsequently, the patient either did not have a high-pressure distal colostogram or had a colostogram without high pressure, and in both circumstances the surgeon “confirmed” his erroneous belief that he was dealing with a case of “high” anorectal malformation. Because of that, he went ahead with an abdominoperineal operation designed for the treatment of more complex malformations, an operation that is not indicated in these cases, and that leaves the patients fecally incontinent. This is something tragic that should never occur if one follows specific steps in the diagnosis of these defects.
The perineum of these patients may have different external appearances. Sometimes they have what is called “bucket handle” malformation. Figure 8.11 shows three different variants of a “bucket handle” malformation. Below the strip of the skin (bucket handle), one can find the fistula orifice. Sometimes, the orifice is located at the base of the penis (Fig. 8.12). In other cases the fistula runs subcuticular in the midline raphe, forming a black ribbon type of structure, which represents the presence of meconium running in a subepithelial tunnel (Fig. 8.13). Other times, we do not see a black ribbon type of structure but rather a white ribbon which represents mucous in the subepithelial fistula (Fig. 8.14). The treatment in these patients consists in unroofing that tract until we reach the real fistula and then to continue with the technique described in this chapter.
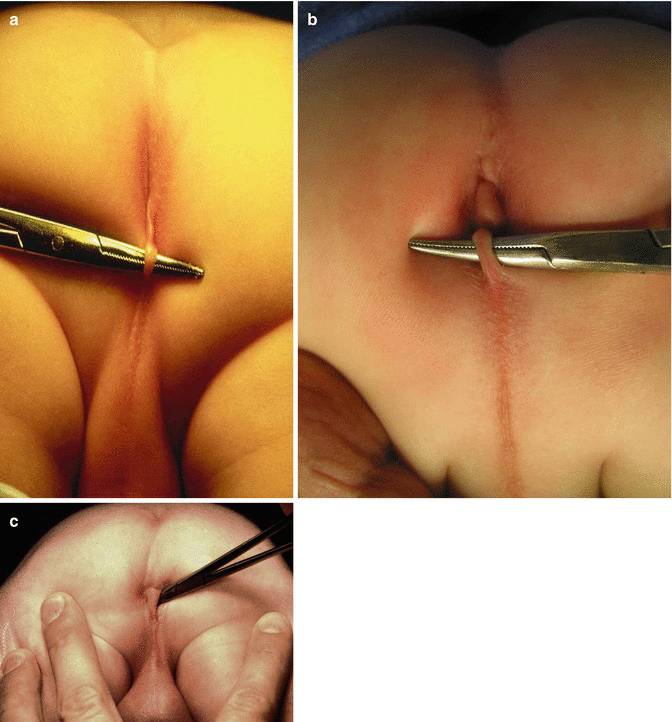
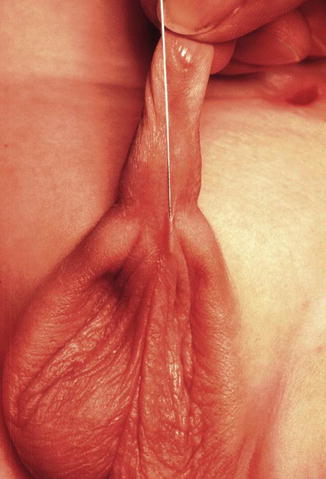
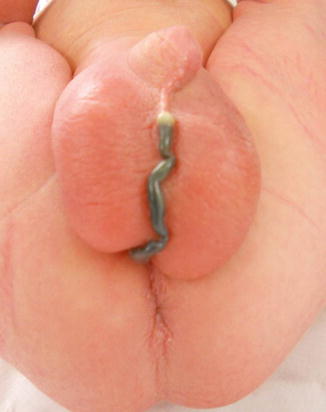
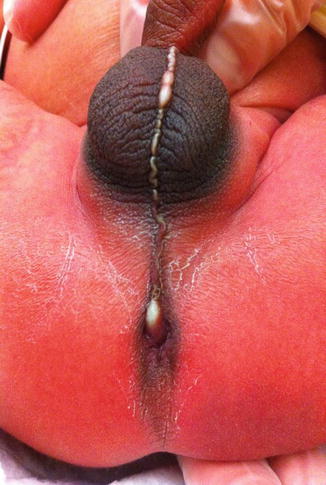

Fig. 8.11
Bucket handle malformations. Three different variants (a–c)

Fig. 8.12
Perineal fistula located at the base of the penis

Fig. 8.13
“Black ribbon” malformation. Variant of a perineal fistula (subepithelial with meconium)

Fig. 8.14
“White ribbon” malformation (subepithelial with mucus) – variant of a perineal fistula
One must be aware of the fact that sometimes, some patients are born with a fistula located at the base of the penis or in the penis itself, and one believes that it runs at a subcuticular level until it reaches the rectum, and therefore, it can be treated like a regular perineal fistula. Yet, surprisingly, one may find that the fistula runs rather parallel to the urethra, deeper and deeper in to the perineum, and through the corpora (Fig. 8.15). As a consequence, trying to follow that structure, the surgeon may provoke significant bleeding. Actually, these specific variants are exceptions to the rule. These malformations are considered rather complex because they require more mobilization of the rectum as well as to be separated completely from the urethra. Fortunately, in most of the cases, one can say that if one is able to see a perineal orifice passing meconium, it means that we are authorized to operate without a colostomy in the way that it was described.
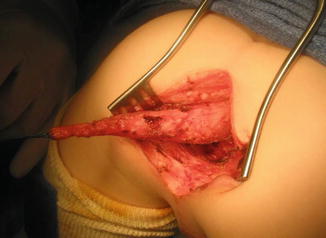

Fig. 8.15
Operative picture of a long narrow perineal fistula. Very rare variant
A sacral defect seen in an anterior/posterior view of an x-ray film (Fig. 8.16) is always associated to a presacral mass [38], and the defect can be very small (Fig. 8.17) or giant (Fig. 8.18). It can be located laterally, giving the impression of a “hemisacrum” or “scimitar.” It can also be located in the midline (Fig. 8.19) giving an image of a “bifid” sacrum. The size and location of the sacral defect usually equals the location and size of the mass. An MRI study will provide more accurate information about the characteristics of the mass, as well as the possible communication with the dural space (anterior meningocele) (Fig. 8.20). The most common types of presacral masses are, by far, teratoma/dermoid, lipoma, meningocele, or a combination of all of these. Presacral masses associated to perineal fistulas or to “rectal stenosis” represent an excellent example of the importance of having a good index of suspicion. The presence of the mass may dramatically change the functional prognosis for bowel and urinary function, particularly if the sacral defect is large. In other words, a perineal fistula with normal sacrum has an excellent functional prognosis, yet the presence of a mass is a rather devastating finding. The surgical strategy also changes, since it must include the resection of the mass at the same time of the repair of the anorectal malformation. This resection can be a rather easy procedure or can also be a formidable operation that requires the participation of a neurosurgeon.
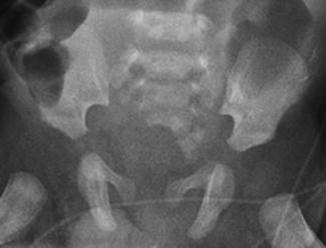
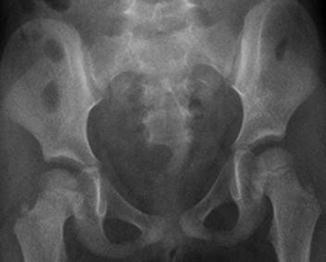
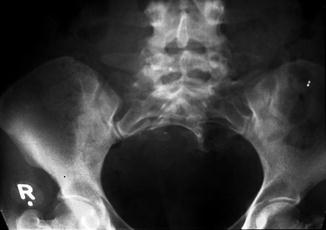
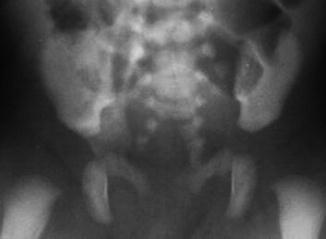
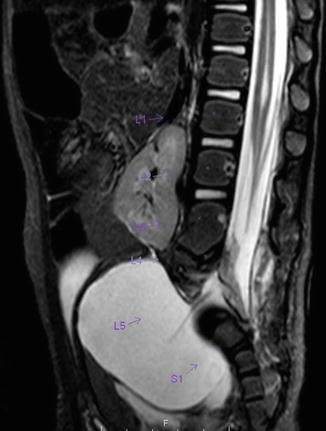

Fig. 8.16
Hemisacrum

Fig. 8.17
Small sacral defect

Fig. 8.18
Giant sacral defect. The size of the presacral mass corresponds to the size of the sacral defect

Fig. 8.19
Bifid sacrum, the mass is located in the midline

Fig. 8.20
MR image of a presacral mass with an anterior meningocele
Some patients are born with a normally located anus and a presacral mass producing a rectal stenosis (Fig. 8.21). The perineal appearance of these patients deserves a special comment. This type of perineum is known as “funnel anus” (Fig. 8.22). It certainly looks like a funnel. The pectinate line is not visible outside because it seems to be located higher, by the pushing effect produced by the mass. This external appearance must prompt us to order an AP x-ray film of the sacrum and an MRI of the pelvis, in order to confirm the diagnosis of the presacral mass and rectal stenosis.
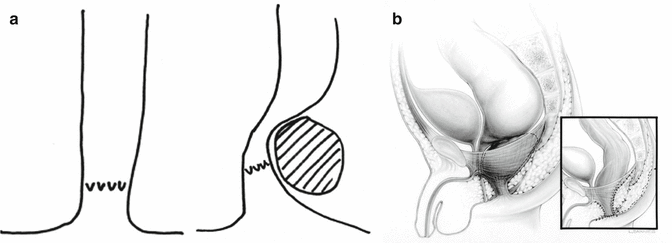
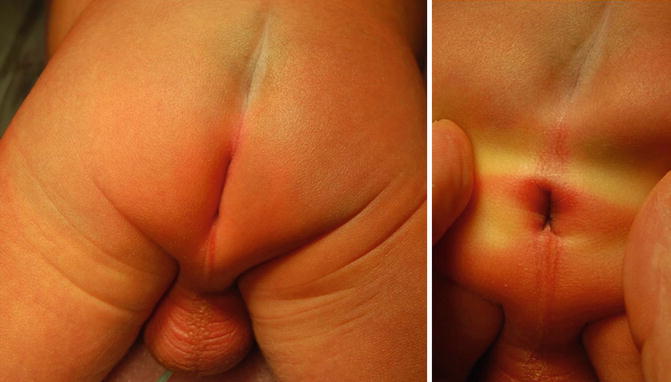

Fig. 8.21
Presacral mass producing a rectal stenosis. (a) Diagram showing the rectum compressed by a presacral mass, which pulls up the anal canal. (b) Diagram of a perineal fistula associated to a presacral mass (pre- and postoperative)

Fig. 8.22
Picture of the perineum of a patient with a “funnel anus”
8.4 Management
Since these defects represent the simplest of all anorectal malformations, the operation designed to repair these defects is also a limited, rather simple, yet meticulous operation. Operations to repair these defects are performed without a protective colostomy. The exceptions to this rule include cases of misdiagnosis. More specifically, some male patients are born with no anal opening; they rather have a tiny perineal orifice that goes unrecognized, and the surgeon erroneously believed that the patient had a “high” malformation and opens a non-indicated colostomy.
When the patients are born full term at our institution, with no serious associated defects, we operate on them without bowel preparation within the first 72 h of life. Unfortunately, however, patients frequently come to us weeks or months after they were born, and in that type of case, we feel it is safer to prepare the gastrointestinal tract with GoLYTELY as described in the chapter related to colonic preparation. In the case of a newborn operated in the first hours of life, we feed the patient usually 3–5 days after the operation. We believe that those babies operated early, without bowel preparation, have a better postoperative course and less chance of infection, perhaps due to the fact that the bacterial proliferation in the bowel is nil or very limited. However, in older patients, we are stricter in the protocol of management. We use GoLYTELY for total bowel preparation (see Chap. 7), insert a central line, and keep the patients 10 days with nothing by mouth.
During the newborn period, there are several therapeutic options for these patients. The first one is simple dilatation of the perineal orifice.
8.5 Dilatations
Dilatations usually help the patient to eliminate the stool and avoid abdominal distention. This treatment modality is preferred by many doctors all over the world, based on the fact that these patients will have bowel control with and without an operation. In fact, misadventure and catastrophic events in these patients occur when somebody performed the wrong type of operation or technically incorrect procedure that ended up in a series of complications that will be described later. Therefore, we can say that it is preferable for the patient to receive anal dilatations rather than a poor operation. The fact is that these patients will have bowel control with and without an operation. We explain that, by the fact that the mislocation of the anal orifice is only present in the lowest part of the rectum (Fig. 8.2), most of the rectum, however, is well located traversing within the funnel-like muscle structure and is only deviated in the lowest part. It is this type of case that exemplifies the fact that a perfect location of the anus, within the sphincter mechanism, is not a precondition to have bowel control. Bowel control then seems to depend on many other factors besides anal location.
Anal dilatations, from our point of view, are then indicated under specific circumstances. One of those could be a patient that has multiple serious associated defects and abdominal distention. Premature babies with severe cardiac conditions that are not in good condition to be taken to the operating room are also good candidates to receive anal dilatations. The protocol of dilatation should be as the one described here (Chap. 18). Another reason to do dilatations is the case in which the surgeon has no experience with performing a formal posterior sagittal anoplasty for these patients. In that case, the chances of hurting the patient are much less with anal dilatations than with an operation. The operation also could be done later. Anal dilatations in cases of perineal fistula are sometimes rather difficult and painful. The reason is that one has to dilate a congenital stricture type of anomaly. This is not the same as in cases of postoperative dilatations after a technically correct operation; in those cases usually the dilatations are uncomfortable, but not painful. Very painful dilatation usually means a congenital stricture or ischemia that occurred during the performance of an anoplasty.
8.6 Cutback Operation
The cutback operation is another therapeutic alternative for these patients. The indications are similar to the ones of the anal dilatation. In fact, it is a procedure that can be done with local anesthesia. Again, we do not think that it should be the final ideal operation for these patients, but it is certainly an alternative for patients who are extremely sick or in situations in which the surgeon does not have the necessary training to perform a formal posterior sagittal anoplasty. The cutback procedure consists of making an incision in the posterior rim of the anal opening and suturing the skin to the mucosa. In other words, it is a kind of Heineke-Mikulicz type of procedure. The cosmetic appearance after the cutback is less than optimal, yet this operation does not really hurt the sphincter mechanism or the innervation of the bowel, and therefore it is considered a good contemporizing preliminary operation, designed to facilitate the passing of stool and alleviate the abdominal distention.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








