Fig. 11.1
Rectal prolapse
Evacuatory dysfunction frequently occurs with rectal prolapse and fecal incontinence has been found in approximately 50–75 % of patients with rectal prolapse [1, 4]. Several factors may contribute to this association: traumatic stretch of the sphincter complex by the protruding rectum or continuous stimulation of the rectoanal inhibitory reflex by the prolapse resulting in chronic low internal anal sphincter pressures and the presence of a direct conduit bypassing the sphincter mechanism [1, 4]. Interestingly, constipation is also a commonly associated feature of rectal prolapse, reported by 25–50 % of patients [4]. Although the mechanism is not clear, it appears to be related to difficulty with defecation due to an obstructing rectal intussusception, paradoxical puborectalis contraction, or colonic dysmotility [1, 4].
Diagnosis and Evaluation
Key Concept: Rectal prolapse is a clinical diagnosis. Underlying symptoms and risk factors should guide the evaluation.
History and physical examination are the key components to the diagnosis of full-thickness rectal prolapse. History and symptoms including persistent or spontaneous prolapse, prolapse with straining, and other symptoms including rectal pain, bleeding, mucous secretion, fecal incontinence, and constipation should be ascertained. Previous surgical history including colon resections or anorectal surgery and anorectal congenital anomalies repaired during childhood must be documented, as well as medical comorbidities. During the physical examination, reproduction of the prolapse with Valsalva should be performed; status of the anal sphincters, perineal descent, and associated pelvic organ prolapse should also be documented. If the prolapse is not reproduced in the lateral decubitus or prone positions in the office, the patient should sit on a commode and strain.
Adjunctive imaging and testing are not always necessary, but may be helpful. If the prolapse cannot be detected in the office, defecography should be performed. This procedure will also identify some associated pelvic floor disorders or defects that may require treatment as well [4]. A lifelong history of severe constipation should be investigated with colonic transit study and defecography to evaluate for colonic inertia or puborectalis dysfunction; these findings may change the planned operation (total abdominal colectomy with rectopexy) or aid in postoperative planning for biofeedback. Colonoscopy should be undertaken before operative intervention according to guidelines for colorectal cancer screening and surveillance as well as to exclude any findings that may change the surgical approach (incidental colon cancer or a tumor serving as lead point for rectal intussusception) [4]. Anorectal physiology testing may not be helpful since studies have shown that these tests do not reliably predict postoperative function after prolapse repair [4].
Types of Operative Repair
Key Concept: Understanding the risks, benefits, and technical details of the various approaches for rectal prolapse repair will aid in proper selection of operative repair and help optimize outcomes. In general, functional improvements for both constipation and fecal incontinence occur following both perineal and abdominal repair.
Perineal Operations
Key Concept: Perineal approaches are a second resort, normally to be used only in patients with medical comorbidities that prevent abdominal approaches. Your preferred choice is almost always to perform an Altemeier outside of mucosal or limited full-thickness prolapse where a Delorme may be useful.
Two main perineal operations are currently performed: the Altemeier (perineal rectosigmoidectomy) with or without levatorplasty and with or without colonic J pouch and the Delorme. Anal encirclement procedures, including the Thiersch operation, have for the most part been abandoned due to high complication rates (infection, erosion, breakage) and high recurrence rates [1]. The Delorme procedure, first described in 1900, is performed most commonly for mucosal prolapse or short full-thickness rectal prolapse [1, 4]. This operation involves circumferential mucosal sleeve resection of redundant mucosa with imbrication of the muscularis layer and then mucosal anastomosis [4]. The complete bowel wall is not resected. This perineal operation has been shown to improve incontinence when performed with or without a sphincteroplasty [1]. It may be technically more challenging than the Altemeier and has been reported to have higher recurrence rates as well [1]. Although complications are thought to occur less frequently than with the abdominal approach, urinary retention, infection, bleeding, and fecal impaction have been reported in 4–12 % [4]. Patients will usually have postoperative improvement of their fecal incontinence (25–70 %) and constipation (13–100 %); however, urgency and tenesmus may occur in a small percentage of patients [4].
Auffret in France first described perineal rectosigmoidectomy in 1882; a series of six cases were published by Mikulicz in 1889 [7]. Miles popularized the technique in 1933, although it was named after Altemeier, who described the technique with an associated levatorplasty in 1971, reporting a recurrence rate of only 2.8 % [2]. This operation involves transanal full-thickness resection of the rectum and sigmoid colon (Figs. 11.2 and 11.3). A coloanal anastomosis is then created with sutures or staples [4] (Fig. 11.4). Some surgeons reserve this perineal repair for older patients with comorbidities since major complications are reported to occur less often than after abdominal operations; the procedure can be performed without general anesthesia, and hospital stay is shorter [4]. Complications do occur, including pelvic bleeding, anastomotic leak, abscess, stricture, and bleeding from the anastomosis, but rates are typically less than 12 % [1, 4]. In addition, complications related to abdominal operations and pelvic dissection, such as sexual dysfunction, small bowel obstructions, wound infections, and incisional hernias, are avoided with the perineal approach [7]. Most studies show improvement in continence after perineal proctosigmoidectomy with levatorplasty similar to that seen after abdominal operations (20–90 %) [1]. Functional improvement in constipation is reported in a smaller number of series, with rates ranging from 61 to 100 %. The addition of the levatorplasty appears to decrease short-term recurrence rates and prolong the recurrence-free interval [1]. The addition of the colonic J pouch may also improve postoperative function. The senor author first described it and routinely employs it [8]. Some surgeons report low recurrence and morbidity rates with the perineal approach and advocate the Altemeier as the first-line treatment for patients of all ages.
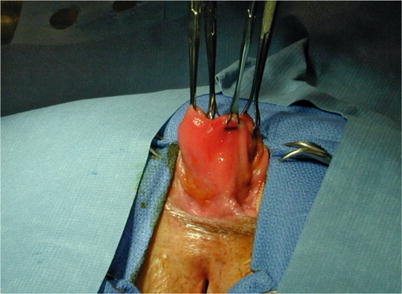
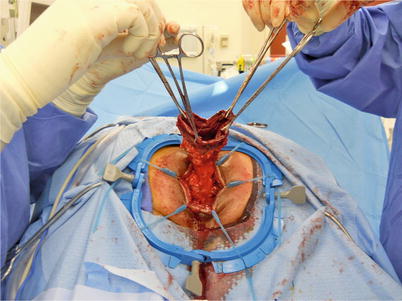
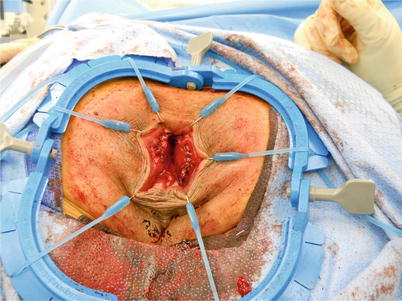

Fig. 11.2
Rectal prolapse during perineal repair

Fig. 11.3
Extraction of rectum and sigmoid during perineal proctosigmoidectomy

Fig. 11.4
Hand-sewn coloanal anastomosis during perineal proctosigmoidectomy
Abdominal Operations
Key Concept: An abdominal operation, through either an open or minimally invasive approach, is the preferred operation for full-thickness rectal prolapse if the patient’s risk profile permits. Regardless of the method chosen, adequate mobilization and fixation at the sacral promontory are required for optimal outcomes.
A variety of abdominal operations are performed to treat rectal prolapse based on the general principles of rectal mobilization and suspension of the rectum out of the pelvis. The operations vary by surgical technique (mobilization only, mobilization-rectopexy, mobilization-resection-rectopexy), means of access (laparoscopic or open) and method of fixation (suture or mesh) [9]. The extent of dissection, division of the lateral stalks, need for concomitant sigmoid resection, and method of rectal fixation are variable and remain points of controversy, as no single method has been shown to be more successful than the others and all have associated failures. Laparoscopy has been increasingly used for a variety of the prolapse repairs (including mesh or suture rectopexy and/or sigmoid resection) with similar recurrence and postoperative incontinence and constipation rates when compared to open surgery. Studies evaluating the laparoscopic approach indicate longer operative times but shorter hospital stays and lower overall costs [1].
Mobilization of the rectum should extend caudally to the level of the pelvic floor musculature, specifically to the levator ani muscles, while the extent of lateral mobilization remains controversial (Fig. 11.5). In a small study performed by Speakman et al., division of the lateral stalks appeared to be associated with a high rate of postoperative constipation; however, division of the stalks was also found to be associated with decreased recurrence [1, 4]. Other studies have shown higher rates of constipation with stalk preservation [4]. Currently, most surgeons preserve the lateral stalks or may divide one lateral ligament.
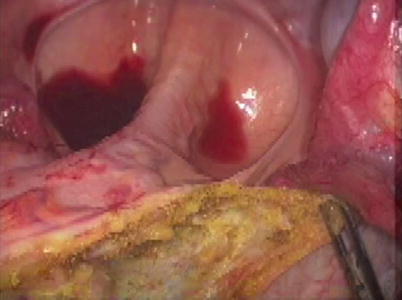

Fig. 11.5
Rectal mobilization during laparoscopic rectopexy
The method of fixation of the rectum is also controversial, manifesting in the multitude of techniques available. Suture rectopexy is commonly performed with the advantage of avoiding the use of a prosthetic mesh, hopefully allowing good fixation with very little risk of infection or erosion, especially if a resection is performed concomitantly. Fixation with tacks has also been used. Rectopexy with mesh has been described with anterior or posterior placement [4]. The Ripstein repair and its variations involve placing a synthetic mesh around the mobilized rectum, attaching the mesh to the presacral fascia below the sacral promontory. Studies have shown low recurrence rates as well as improved incontinence in 20–60 % of patients [4]. Erosion and defecation problems have been noted with the anterior repair, and, accordingly, alternative approaches (attachment to the lateral mesorectum or variety of mesh types) have been used. The Wells mesh rectopexy originally used an Ivalon sponge (polyvinyl alcohol) with division of the lateral ligaments. The sponge is no longer used due to increased complications and postoperative constipation; however, this technique continues to be used with synthetic mesh [4]. Ventral mesh rectopexy (anterior mobilization of rectum) has also been described by D’Hoore et al., in order to decrease postoperative constipation. Orr-Loygue mesh rectopexy mobilizes both the anterior and posterior rectum before fixation with mesh. Studies show low recurrence rates (3.4 %) and decreased postoperative constipation with ventral rectopexy; however, the rate of new-onset constipation has been reported at approximately 14 % [4]. To date, no randomized trials have compared suture rectopexy to mesh fixation.
Concomitant sigmoid resection performed with suture rectopexy was first described by Frykman and Goldberg in 1969 [1]. The addition of the resection has decreased rates of postoperative constipation, but it does not appear to affect recurrence rates. The addition of a resection to the operation increases the potential risks associated with the transection of bowel and creation of an anastomosis, including anastomotic leak, abscess, and wound infection.
Laparoscopy and Rectal Prolapse Repair
Key Concept: A minimally invasive approach provides similar outcomes as open surgery, given experience and technical proficiency, and is increasingly being performed via laparoscopic and robotic means.
The first laparoscopic repair of rectal prolapse (rectopexy) was performed in 1992 [4]. Since that time, several studies have demonstrated that laparoscopy is safe and feasible for the treatment of prolapse (Figs. 11.6 and 11.7). Although these studies are small, they have shown similar recurrence and morbidity rates (4–8 % and 10–33 %, respectively) when compared to the open approach [4]. The benefits of laparoscopy have been reported in several studies. One small randomized trial by Solomon et al. found laparoscopy to be associated with less postoperative pain, faster return of bowel function, and shorter hospital stays, although operative times were longer [4, 10]. A meta-analysis performed in 2005 compared open to laparoscopic abdominal rectopexy [3]. This group found that operative time was longer for laparoscopy, but length of stay was shorter. However, overall recurrence was found to be similar between open and laparoscopic abdominal rectopexy with a mean follow-up time of 12–31 months for the included studies [3]. Byrne et al. investigated long-term functional outcomes between laparoscopic rectopexy and open and resection-rectopexy. They found that the recurrence rate remains low (7 % at 5 years and 11 % at 10 years follow-up) and that improvement of postoperative incontinence and constipation is similar when compared to the other operations; the majority of patients felt as if their constipation improved after surgery, and incontinence scores had improved [11]. The rate of wound complications also appeared to be lower in the laparoscopic group [4].
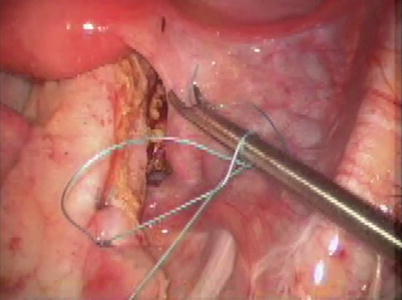
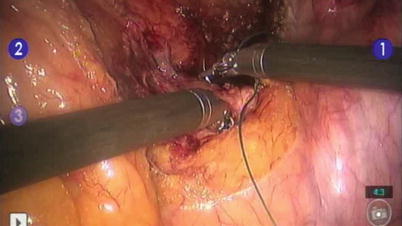

Fig. 11.6
Laparoscopic suture rectopexy

Fig. 11.7
Suture placement during robotically-assisted suture rectopexy
Robotic surgery has also been applied to the treatment of rectal prolapse (Fig. 11.7). There have been only a few series with small numbers reported. One small nonrandomized trial reported longer operating time and higher costs, but robotic-assisted mesh rectopexy was found to be safe and feasible [12]. Nonetheless, the visualization and ease of suturing during robotic surgery appears improved compared to conventional laparoscopy [4, 12]. Non-resectional rectopexy may be one of the better indications for robotic surgery as the procedure requires only a single docking of the robot and no incision is necessary for specimen retrieval. Moreover, unlike using laparoscopic instruments to suture the rectum to the anterior sacral fascia and periosteum, this step may potentially be reliably undertaken with the robotic platform.
Recurrence After Initial Repair
Key Concept: Recurrence rates increase with longer follow-up intervals and are typically higher with perineal procedures. An abdominal repair should be the initial approach when the patient’s risk profile permits, while the type of procedure (i.e., rectopexy alone, mesh, resection) does not seem to influence recurrence rates.
Recurrence after initial prolapse repair has been reported to occur in 2–60 % of patients. These recurrence rates vary with type of approach, technique, preoperative anorectal and pelvic anatomy and function, and length of follow-up among other variables. Most studies have shown lower recurrence rates for abdominal repairs; whereas the reasons for this difference are not exactly clear, it is likely due to inadequate mobilization and resection of the rectum and sigmoid that results from lack of direct visualization during the perineal approach. Several studies have tried to identify predictors of recurrence. To date, there is very little conclusive evidence identifying specific risk factors. It is possible that rectal prolapse is just one symptom of overall pelvic floor dysfunction, which may recur if the overall pelvic environment is not changed.
Recurrence After Altemeier Procedure
Key Concept: The wide range of reported recurrence rates following an Altemeier are likely multifactorial and include technical variables (i.e., small resected segment, failure to enter the peritoneal cavity) and patient-driven factors (prior surgery, length of follow-up).
Several studies have examined the recurrence rates after perineal rectosigmoidectomy. In a literature review, the range of recurrence rates found for the Altemeier procedure was between 0 and 58 % [2]. In a study performed by Altomare et al., 17 (18 %) of 93 patients experienced complete recurrence after being followed for at least 12 months (median 41, 12–112). Six other patients had recurrence of mucosal prolapse only [2]. Of the 17 patients with full-thickness recurrence, repeat Altemeier was performed in 6, Delorme in 1, and Wells mesh rectopexy in 1; the 9 patients refused further surgery [2]. These authors found that only previous surgery for prolapse was associated with higher recurrence; other factors such as duration of follow-up, length of resected specimen, levatorplasty, age, sex, and severity of incontinence were not significantly associated [2]. At Washington University in St. Louis, Glasgow et al. found a recurrence rate of 8.5 % when 106 consecutive patients with full-thickness rectal prolapse had perineal proctectomy performed regardless of preoperative status (age, comorbidities, previous abdominal or anorectal surgery). In a study by Cirocco et al. published in 2010, 103 consecutive patients with full-thickness rectal prolapse were treated with perineal proctosigmoidectomy between 2000 and 2009. The authors reported that 61 % of patients had preoperative constipation; 94 % of these patients had improvement. Forty-seven percent had fecal incontinence, which improved in 85 % of patients following surgery [7]. This group found no recurrences with a mean follow-up of 43 months [7]. In their discussion, the authors allude to their lack of understanding regarding the historically high recurrence rates reported after perineal repair. Interestingly, in their literature review, cumulative recurrence rate was 37 % for studies performed before 1971 and only 10 % in reports published after 1971 [7]. The higher rates of recurrence were suspected to be due to poor surgical technique, especially in those cases where the mobilization failed to enter the peritoneal cavity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








