Radiology of the Urinary Tract: Introduction
The pace of innovation in diagnostic radiology has increased exponentially, in tandem with computer advances and the rapid evolution of microprocessing power. Imaging of the urinary tract, as a result, has become more flexible and precise, with new procedures offering a great selection of options, and new imaging algorithms being implemented. Ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI) provide higher soft-tissue contrast resolution than conventional radiography, as well as multiplanar imaging capability, resulting in significant advances in almost all areas of uroradiology. In academic centers, metabolic and molecular imaging techniques have become the focus of new research, and have begun to enter the realm of daily clinical practice. While imaging advances have produced new algorithms for approaching diagnostic evaluation, appropriate use of imaging in each particular case also depends greatly on the equipment and professional talent available. One imaging modality or protocol may offer specific advantages over another depending on the clinical question, and the importance of a collaborative approach from the medical team cannot be overemphasized.
In summary, ever changing uroradiology remains indispensable in the diagnosis and treatment of patients with urologic disorders. This chapter will discuss the imaging techniques used in uroradiology, with summaries of the advantages and disadvantages of the various techniques, and will end with a brief discussion comparing imaging methods.
Radiography
X-rays are electromagnetic waves with photon energies that typically fall between those of gamma rays and ultraviolet radiation. Radiography is possible because tissues differ in their ability to absorb x-rays. A radiopaque contrast medium is frequently employed to enhance soft-tissue contrast.
Although newer imaging techniques have largely replaced conventional radiography for diagnosis of many urologic problems, general radiography remains useful for some urologic disorders; therefore, the urologist should be familiar with x-ray equipment and uroradiologic techniques. The basic types of uroradiologic studies are plain (conventional) abdominal films, (also known as KUB, which stands for kidney, ureter, bladder) intravenous urograms (IVUs), cystourethrograms, urethrograms, and angiograms. These studies are described separately in sections that follow.
Many conventional x-ray units contain both radiographic and fluoroscopic capabilities. These require a high voltage power supply, an x-ray tube, a collimating device, and an x-ray detector or film. Fluoroscopic units also use an electronic image intensifier and an image display system. Today, more radiology departments have become completely “filmless” as digital recording, displaying, and archiving of images are replacing film-based techniques.
Image intensifiers, coupled to video cameras, electronically augment the ordinary dim fluoroscopic image.
Conventional recording of an x-ray image uses film and intensifying screens. The image intensifier and camera can be used to capture dynamic and static images. Real-time images are now typically recorded using conventional or digital video. Conventional spot or cine images may be acquired on x-ray film or digitally recorded.
Radiographic contrast media used in uroradiography are water-soluble iodinated compounds that are radiopaque. Similar compounds are used for basic radiographic techniques and CT, though iodine concentrations will differ depending on preference and route of administration. In general, intravenous administration for CT or IVU is performed with iodine 200 mg/lb body weight in adults, and direct instillation to the collecting system or bladders uses similar media diluted to 15–45% concentration. The extracellular distribution of these agents results in improved contrast resolution and conspicuity of various structures.
Significant advances in water-soluble contrast media occurred with the introduction of low-osmolality, nonionic organic iodine-containing compounds. These nonionic agents significantly improve patient tolerance and decrease the incidence of adverse reactions, and at many institutions the use of nonionic agents is standard. Whether they reduce the mortality associated with the use of contrast media has not been proven. The major obstacle to their use is higher cost.
All procedures using intravascular contrast media carry a small but definite risk of adverse reactions. The overall incidence of adverse reactions is about 5%. Reactions in nonintravenous use (ie, cystograms) are extremely rare but have been reported.
Most reactions are minor, for example, nausea, vomiting, hives, rash, or flushing, and usually require only reassurance. Cardiopulmonary and anaphylactoid reactions can occur with little warning and can be life threatening or fatal. In a large meta-analysis, the incidence of death due to intravascular injection of contrast media was 0.9 deaths/100,000 injections. There are no reliable methods for pretesting patients for possible adverse reactions. The risks and benefits of contrast use should be carefully evaluated for each patient before the procedure is initiated. Treatment of adverse reactions involves the use of antihistamines, epinephrine, vascular volume expanders, bronchodilators, and other cardiopulmonary drugs as well as ancillary procedures indicated by the nature and severity of the reaction. In some cases a radiographic examination using intravascular contrast media is critical even if the patient has had a prior moderate or severe reaction. Such patients are given nonionic contrast agents and pretreated with corticosteroids, sometimes in combination with antihistamines, in an effort to prevent recurrence. This preventive treatment is not always successful, so any decision to administer contrast under these circumstances should be carefully weighed against the risks.
Nephrotoxicity caused by intravascular contrast agents is another concern. The pathogenesis of contrast nephropathy (CN) likely involves medullary ischemia due to contrast-induced vasoconstriction and direct tubular injury. Patients at higher risk are those with preexisting renal insufficiency, diabetes, dehydration, or patients who receive higher volumes of contrast material. Alternative procedures can be selected in high-risk patients. If contrast use is deemed necessary in a high-risk individual, CN can be minimized through attention to proper hydration, discontinuation of drugs that may exacerbate toxic effects, adequate hydration in the 24 hours prior to scanning, reduction of contrast volume, and possibly administration of oral N-acetylcysteine.
Radiography produces anatomic images of almost any body part. Costs are moderate compared with cross-sectional imaging systems. Space requirements are modest, and portable equipment is available for use in hospital wards, operating rooms, and intensive care units. Because there are a great many specialists trained in radiography, its use is not confined to large medical centers. The major disadvantage of radiographic imaging is the use of ionizing radiation and relatively poor soft-tissue contrast. The evaluation of the urinary tract almost always requires opacification by iodine contrast media.
A plain film of the abdomen, frequently called a KUB film, is the simplest uroradiologic examination. It is generally the preliminary radiograph in extended radiologic examinations, such as intravenous urography, and is usually taken with the patient supine. It may demonstrate osseous abnormalities, abnormal calcifications, or large soft-tissue masses.
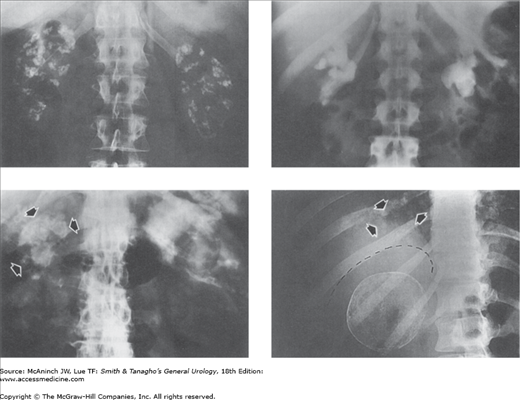
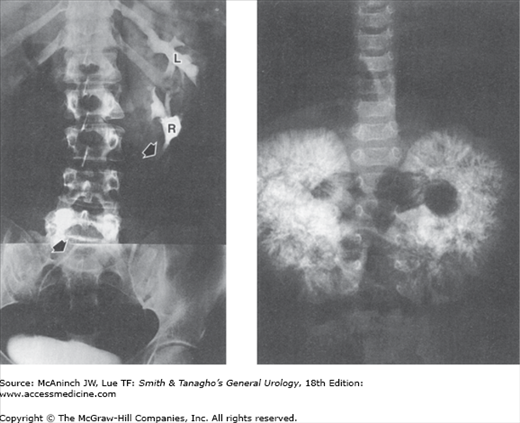
Figure 6–1.
Plain films of the abdomen with abnormal radiopacities. Upper left: Bilateral nephrocalcinosis. Young adult male with renal tubular acidosis. Upper right: Bilateral staghorn calculi. 37-year-old woman with chronic pyelonephritis and history of previous right staghorn pyelolithotomy. Lower left: Renal tuberculosis. Shrunken, autonephrectomized, and calcified right tuberculous kidney (arrows). 74-year-old man with history of renal and thoracolumbar spinal tuberculosis. Lower right: Papillary adenocarcinoma of right kidney. Remarkable tumor surface calcifications. Multiple pulmonary metastases (arrows) from the renal cancer. 22-year-old woman with painless soft tissue mass in the neck.
Figure 6–2.
Plain films of the abdomen with abnormal radiopacities. Left: Schistosomiasis calcification (arrows) in bladder and left ureter. 19-year-old male native of Aden with weight loss and hematuria. Right: Large vaginolith (open arrow) and small, barely visible bladder calculus (solid arrow). 4-year-old girl with common urogenital sinus.
Figure 6–3.
Plain films of the abdomen with abnormal radiolucencies. Left: Emphysematous pyelonephritis. Interstitial striated pattern of radiolucent gas throughout the entire left kidney. Similar changes were present in the right kidney. 58-year-old diabetic man with pyuria and septic shock. Right: Gas pyelogram. No interstitial gas, but gas fills dilated left kidney calices, pelvis, and ureter. 50-year-old diabetic woman with sepsis and left upper urinary tract infection due to gas-forming micro-organisms.
Kidney outlines usually can be seen on the plain film, so that their size, number, shape, and position can be assessed. The size of normal adult kidneys varies widely. The long diameter (the length) of the kidney is the most widely used and most convenient radiographic measurement. The average adult kidney is about 12–14 cm long. In children older than 2 years of age, the length of a normal kidney is approximately equal to the distance from the top of the first to the bottom of the fourth lumbar vertebral body. Patterns of calcification in the urinary tract (Figures 6–1 and 6–2) may help to identify specific diseases.
The collecting structures of the kidneys, ureters, and bladder can be demonstrated radiologically with contrast media by the following methods:
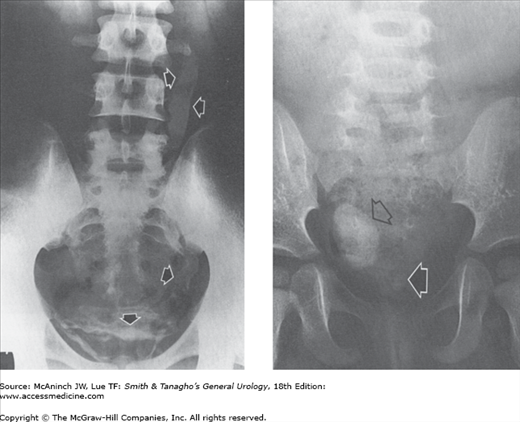
Figure 6–4.
Abnormal excretory urograms. Left: Medullary sponge kidney. Pronounced medullary tubular ectasia (arrows) of entire right kidney. Similar findings were present in upper pole pyramids of left kidney, and small medullary calculi were present in some areas of tubular ectasia in both kidneys. 34-year-old woman with repeated bouts of chills, fever, and left flank pain. Right: Renal tuberculosis. Irregular cavitation of lower pole pyramid (arrow). 22-year-old woman with positive urine culture for tuberculosis.
Figure 6–5.
Abnormal excretory urograms. Left: Crossed fused ectopia. Composite of 2 films from an excretory urogram shows ectopic right kidney (R) fused to left kidney (L). Right ureter (arrows) crosses midline and enters normally into right side of bladder. Healthy 31-year-old female potential kidney donor. Right: Infantile polycystic kidney disease. Very large kidneys with radiopaque spoke pattern radiating out to cortex. 26 hours after administration of intravenous contrast medium. 4-month-old girl with bilateral abdominal masses.
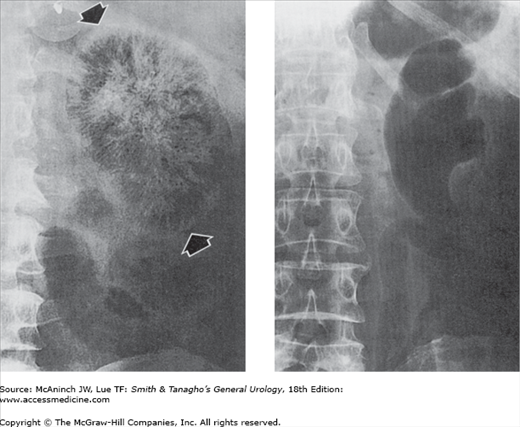
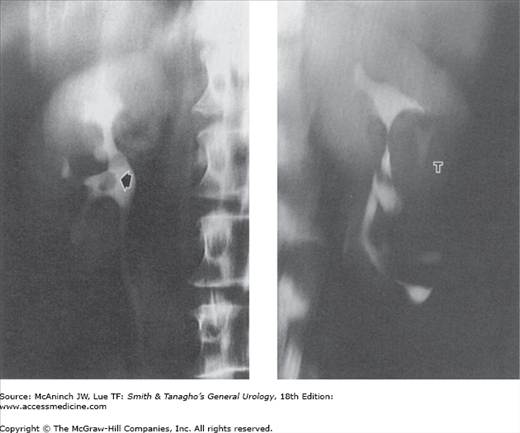
Figure 6–6.
Radiographic tomography. Tomography is used to image a plane in the body. The technique was widely used in uroradiology, often permitting demonstration of lesions otherwise hidden by overlying soft tissues or obscuring bowel shadows. However, computed tomography (CT) is rapidly replacing conventional excretory urograms, and thus tomography is declining in its use as well. Left: Transitional cell carcinoma. The tumor in the pelvis (arrow) is clearly shown free of obscuring gas shadows present on the nontomographic films. 56-year-old man with history of renal calculi. Right: Renal cell carcinoma (T). Displacement of mid-kidney collecting structures and a nephrogram defect are seen free of obscuring splenic flexure fecal shadows that were present on the nontomographic films. 44-year-old woman with fever, weight loss, anemia, and history of contralateral nephrectomy for carcinoma 15 years earlier.
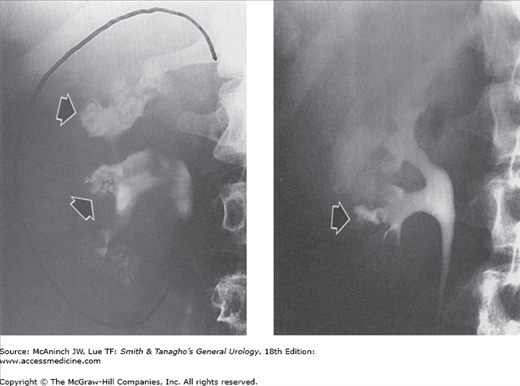
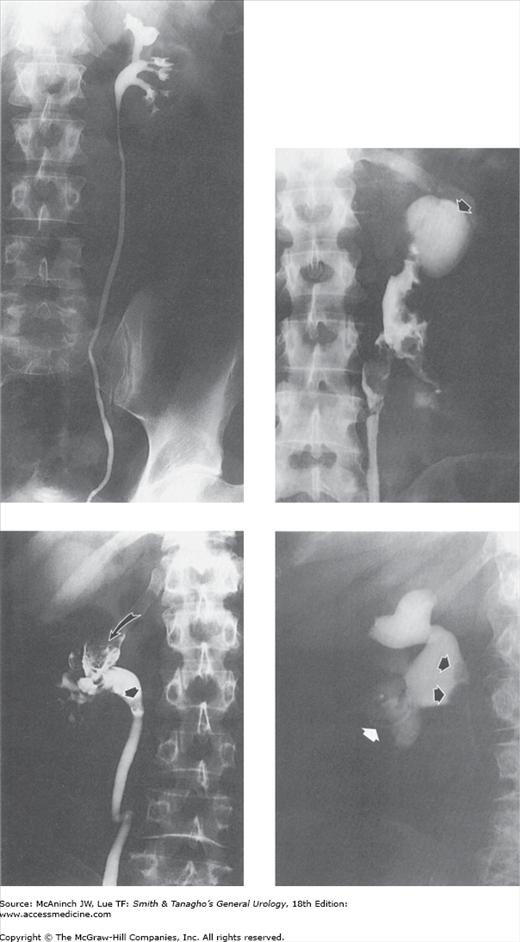
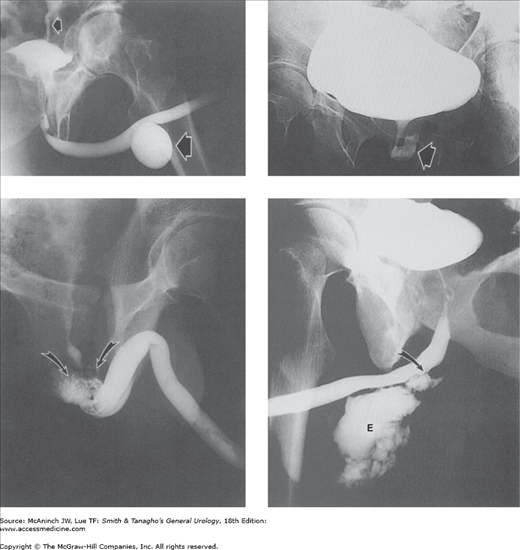
Figure 6–7.
Retrograde urograms and nephrostograms; lower ureters not all shown. Upper left: Normal retrograde urogram. Intrarenal collecting structures, pelvis, and ureter are normal. Adult male with microscopic hematuria and previous technically unsatisfactory excretory urogram. Upper right: Squamous cell carcinoma. Marked irregular filling defects involving calices, pelvis, and proximal ureter, with communicating abscess cavity in upper pole (arrow). Kidney also showed squamous meta-plasia and contained calculi. 51-year-old woman with 2-week history of left flank cellulitis and tenderness. Lower left: Transitional cell carcinoma. Severe deformity with filling defects in right upper pole calices (curved arrow) and blood clots in lower calices and at ureteropelvic junction (straight arrow). 65-year-old man with gross hematuria and right flank pain. Lower right: Fungus balls. Nephrostogram revealing 2 filling defects (arrows) in renal pelvis. Copious fungal matter aspirated through nephrostomy catheter. 65-year-old diabetic woman who had undergone left nephrectomy, with percutaneous nephrostomy catheter (white arrow) for obstruction of right kidney.
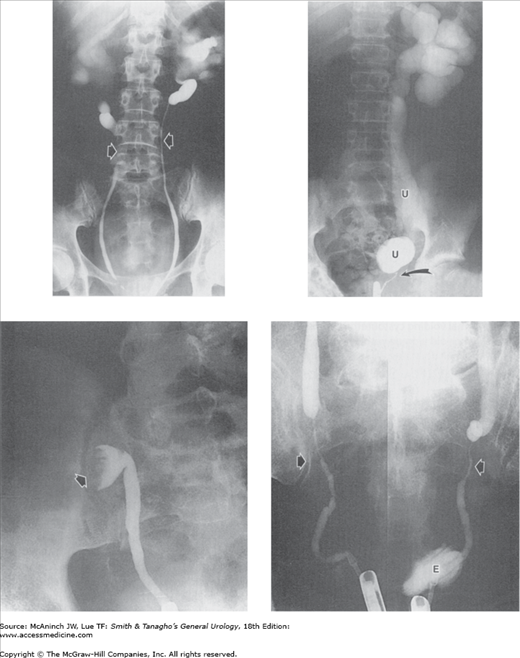
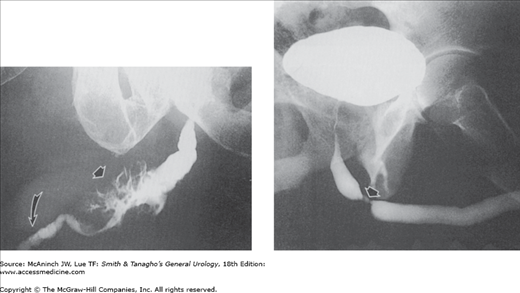
Figure 6–8.
Abnormal retrograde urograms. Upper left: Idiopathic retroperitoneal fibrosis. Smooth narrowing of both mid ureters (arrows), with bilateral proximal ureterectasis and hydronephrosis. 51-year-old woman with no urinary tract symptoms. Upper right: Functional ureteral obstruction. Obstruction was due to congenitally abnormal muscle arrangements in the affected very distal ureter (curved arrow). Pronounced hydronephrosis and dilatation of ureter (U) proximal to the short segment of abnormal ureter. 13-year-old boy with repeated urinary tract infections. Lower left: Transitional cell carcinoma of the ureter. No contrast medium has passed beyond the large, bulky, right ureteral tumor (arrow). The ureteral widening below the tumor is distinctive and is sometimes referred to as the “champagne glass” sign (in this instance, the glass is tipped on its side). 76-year-old man with nonfunctioning right kidney. Lower right: Ureteral constrictions secondary to extension of carcinoma of the colon. Bilateral distal ureteral narrowings (arrows) with upper tract obstruction. Composite of separate retrograde urograms. E = unintended extravasation about tip of left ureteral catheter. 76-year-old man with cancer of the sigmoid colon.
The IVU, also known as excretory urography (EU) (Figure 6–4), or intravenous pyelography (IVP), can demonstrate a wide variety of urinary tract lesions (Figures 6–4 and 6–5), is simple to perform, and is well tolerated by most patients.
CT, sonography and MRI have replaced urography in the vast majority of cases. Nevertheless, urography is occasionally used and is useful for demonstrating small lesions in the urinary tract (eg, papillary necrosis, medullary sponge kidney, uroepithelial tumors, pyeloureteritis cystica).
At one time dehydration was advocated as optimal preparation for intravenous urography (EU). This is no longer required. Furthermore, dehydration is to be avoided in infants, debilitated and elderly patients, and patients with diabetes mellitus, renal failure, multiple myeloma, or hyperuricemia.
It is controversial whether preliminary bowel cleansing is beneficial. The choice may be made according to individual preference.
Following a preliminary plain film of the abdomen, additional radiographs are taken at timed intervals after the intravenous injection of iodine-containing contrast medium. Normal kidneys promptly excrete contrast agents, almost entirely by glomerular filtration.
The volume and speed of injection of the contrast medium, as well as the number and type of films taken, vary by preference, patient tolerance, and the particular clinical scenario.
Radiographic tomography, x-ray imaging of a selected plane in the body, permits recognition of kidney structures that otherwise are obscured on standard radiograms by extrarenal shadows, for example, those due to bone or feces (Figure 6–6). Image-intensified fluoroscopy permits study of urinary tract dynamics. “Immediate” films, which are taken immediately after the rapid (bolus) injection of contrast, typically show a dense nephrogram and permit better visualization of renal outlines. Abdominal (ureteral) compression devices temporarily obstruct the upper urinary tract during EU and improve the filling of renal collecting structures. “Delayed” films, taken hours later or on the following day, can contribute useful information. “Upright” films, taken with the patient standing or partially erect, reveal the degree of mobility and drainage of the kidneys and, if taken immediately after the patient has voided (“postvoiding” film), show any residual urine in the bladder.
Retrograde urography is a minimally invasive procedure that requires cystoscopy and the placement of catheters in the ureters. A radiopaque contrast medium is introduced into the ureters or renal collecting structures through the ureteral catheters (Figures 6–7 and 6–8), and radiographs of the abdomen are taken. This study must be performed by a urologist or experienced interventional uroradiologist. Some type of local or general anesthesia should be used, and the procedure occasionally causes later morbidity or urinary tract infection.
Retrograde urograms may be necessary if excretory urograms or CT urogram (CTU) are unsatisfactory, if the patient has a history of adverse reaction to intravenous contrast media, or if other methods of imaging are unavailable or inappropriate.
Outlining the renal collecting structures and ureters by percutaneous catheter is occasionally done when excretory or retrograde urography has failed or is contraindicated, or when there is a nephrostomy tube in place and delineation of the collecting system is desired. For antegrade studies, contrast medium is introduced either through nephrostomy tubes (nephrostogram) or by direct injection into the renal collecting structures via a percutaneous puncture through the patient’s back. Percutaneous retrograde urograms of the upper urinary tract are made by retrograde injection of contrast medium through the opening of a skin ureterostomy or pyelostomy (skin ureterogram, skin urogram) or through the ostium of an interposed conduit, usually a segment of small bowel (loopogram).
Direct instillation of contrast media into the urinary bladder (cystography) allows a more focused examination of the bladder. Contrast is usually instilled via a transurethral catheter, but when necessary can be administered via percutaneous suprapubic bladder puncture. For urodynamic studies, pressure transducers are used within the bladder lumen and rectum for dynamic measurement of intraluminal and intra-abdominal pressures, respectively. Radiographs can be taken using standard overhead x-rays, or during fluoroscopy. Voiding cystourethrograms are radiographs of the bladder and urethra obtained during micturition. Cystography and cystourethrography are important radiologic techniques for detecting vesicoureteral reflux and may be used in the workup of patients with urinary stress incontinence. CT cystography (CT of the pelvis after the instillation of dilute contrast medium into the bladder) has been shown useful in the evaluation of traumatic bladder rupture.
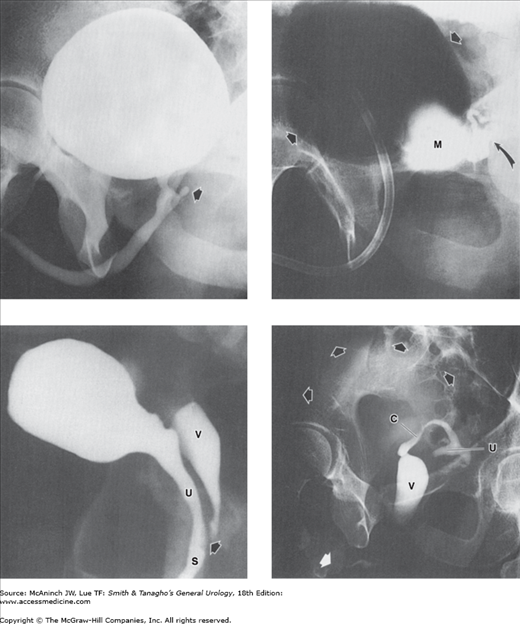
Figure 6–9.
Normal voiding cystourethrograms. Left: Normal female bladder and urethra. Arrow indicates urethral meatus. 22-year-old woman with voiding symptoms. Right: Normal male penile urethra. Large open arrow = prostatic urethra; small open arrow = membranous urethra; closed arrow = penile urethra; curved arrow = verumontanum. 27-year-old man with vague right lower abdominal and testicular pain.
Figure 6–10.
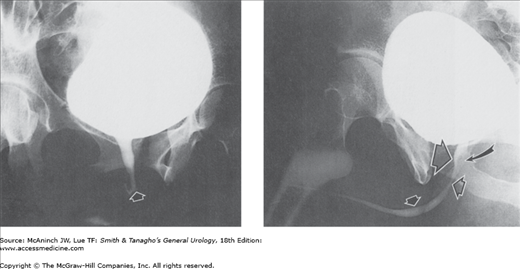
Abnormal cystograms: retrograde cystograms or “cystograms” as part of excretory urogram studies. Upper left: Ectopic ureterocele. Giant ureterocele (straight arrows) to hydronephrotic, nonfunctioning upper portion (curved arrow) of duplex right kidney. 9-month-old-girl with urinary tract infections. Upper right: Pelvic lipomatosis. Pear-shaped bladder and increased radiolucency of the pelvic soft tissues secondary to pelvic lipomatosis of severity sufficient to produce obstructive dilatation of the upper urinary tracts. Filling defects (arrows) at bladder base due to cystitis glandularis. 62-year-old man with intermittent left flank pain. Lower left: Rupture of the membranous urethra. Pear-shaped bladder secondary to extraperitoneal extravasation (E) and perivesical hematoma. Arrow = inflated balloon of Foley catheter. 41-year-old man with renal transplant, after a motor vehicle accident that resulted in pelvic bone fractures, separation of the sacroiliac joints, and dislocation of the left (L) but not the right hip prosthesis (patient has bilateral hip prostheses). Lower right: Bladder hernia. Bilateral obstructive ureterectasis (small arrows) secondary to remarkable herniation of the entire bladder (large arrow, B) into the inguinal region, 5″ 5′, 225 lb, 53-year-old man with panniculus reaching to mid thigh, complaining of difficulty voiding.
Figure 6–11.
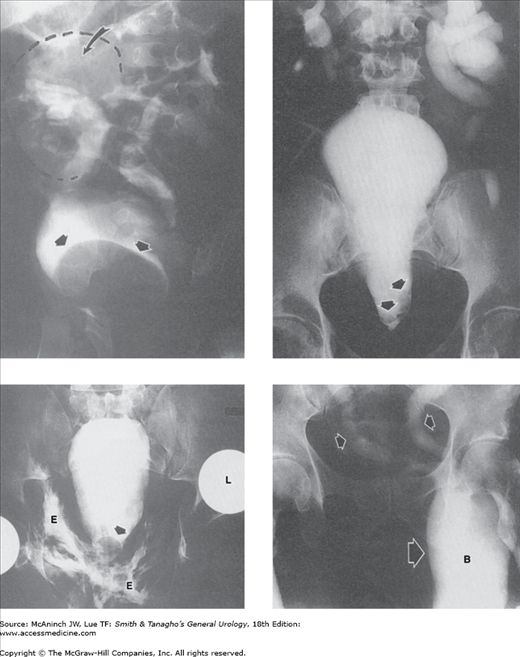
Abnormal cystograms: retrograde cystograms or “cystograms” as part of excretory urogram studies. Upper left: Neurogenic bladder. This neurogenic bladder has a “Christmas-tree” shape, with gross trabeculation and many diverticula. Residual myelographic contrast medium in spinal canal (straight arrow). Right vesicoureteral reflux (curved arrow). 70-year-old man with urinary incontinence. Upper right: Congenital “hourglass” bladder. Transverse concentric muscular band (arrows) separates upper and lower bladder segments, both of which contracted and emptied simultaneously and completely with voiding. 66-year-old woman with urinary stress incontinence. Lower left: Hodgkin’s disease of bladder. Global thickening of the bladder wall (arrows), more apparent on the left. 54-year-old man with generalized Hodgkin’s disease. Lower right: Papillary transitional cell bladder carcinoma. Huge (12 cm) cauliflower-like bladder mass (arrows) filling almost the entire bladder. “Cystogram” film of an excretory urogram in a 40-year-old man with recurrent bladder tumor.
Figure 6–12.
Abnormal prostate and posterior urethra: cystograms and urethrograms. Upper left: Benign prostatic hyperplasia. Gross enlargement of prostate gland producing marked elevation (arrows) of the bladder base. The bladder shows small diverticula and slight trabeculation. Excretory urogram (cystogram) in a 65-year-old man with history of obstructive voiding symptoms. Upper right: Foreign body (eyeliner pencil cover) lodged in bladder and prostatic urethra, with urethrorectal fistula. Radiopaque medium enters rectum and sigmoid colon (S) through fistula (arrow) from prostatic urethra. Retrograde urethrogram in a 43-year-old man. Lower left: Rhabdomyosarcoma of prostate. Lobulated filling defects (large arrow) encroaching on widened prostatic urethra. Voiding cystourethrogram in a 5-year-old boy with voiding difficulties. Small arrow = penile urethra. Lower right: Posterior urethral valves. Marked dilatation and elongation of prostatic urethra (P), with reflux into prostatic ducts (straight arrow) secondary to posterior urethral valves (curved arrow) with bilateral vesicoureteral reflux into dilated ureters (U). Voiding cystourethrogram in a 10-day-old boy.
The urethra can be imaged radiographically by retrograde injection of radiopaque fluid or in antegrade fashion with voiding cystourethrography, or with voiding following EU. The antegrade technique is required when lesions of the posterior urethra, for example, posterior urethral valves, are suspected; the retrograde technique is more useful for examining the anterior (penile) urethra. The urethra may also be evaluated by tailored MRI examinations using thin-section imaging with a small field of view. Urethral tumor or diverticula, for example, may be readily demonstrated with MRI (Ryu and Kim, 2001).
Figure 6–13.
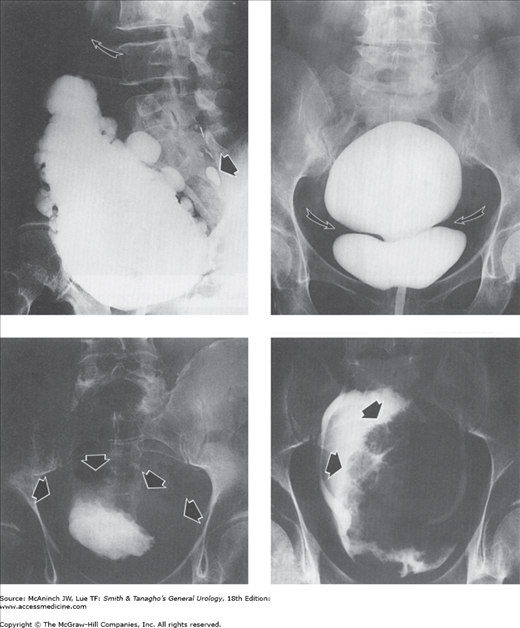
Abnormal anterior urethras: voiding cystourethrograms and retrograde urethrograms. Upper left: Voiding cystourethrogram in a 78-year-old man with a history of urethral diverticulum of unknown etiology. 4-cm anterior urethral diverticulum (large arrow) and left vesicoureteral reflux (small arrow). Upper right: Urethral diverticulum in a woman. Large irregular diverticulum (arrow). Voiding cystourethrogram in a 51-year-old woman with voiding difficulties and suspected urethral stricture. Lower left: Ruptured urethra. Extravasation of contrast medium around the membranous urethra (arrows). Retrograde urethrogram in a 16-year-old boy in whom blunt perineal trauma was followed by bloody urethral discharge and inability to void. Lower right: Urethroscrotal fistula. Extravasation (E) into extraurethral tissues from fistula in bulbous urethra (arrow). Retrograde urethrogram in a 26-year-old man after end-to-end urethroplasty for stricture.
Figure 6–14.
Abnormal anterior urethras: retrograde urethrograms. Left: Urethral carcinoma. Filling of irregular sinus tracts and channels in a large epidermoid carcinoma of the bulbocavernous urethra (straight arrow). There are multiple thin transverse strictures of the penile urethra (curved arrow). 75-year-old man with obstructive voiding symptoms and 30-year history of urethral strictures requiring dilatations. Right: Focal urethral stricture (arrow). Middle-aged man with obstructive voiding symptoms who denied previous urethritis.
Figure 6–15.
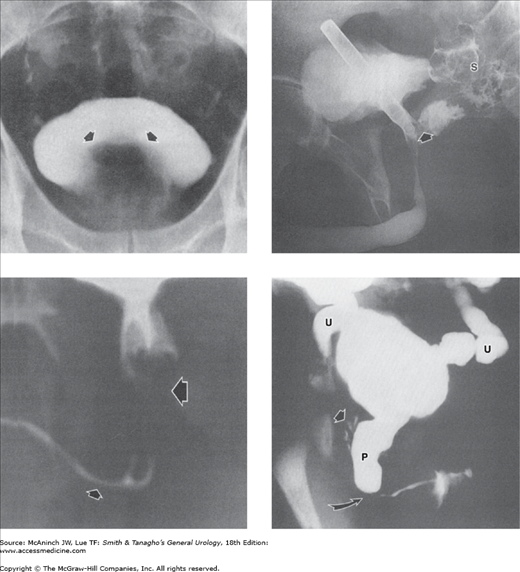
Congenital genitourinary anomalies: voiding cystograms and retrograde urethrograms. Upper left: Utricle. Midline outpouching (arrow) from verumontanum between orifices of ejaculatory ducts, representing Müllerian duct cyst. Upper right: Gas cystogram combined with injection of utricle, oblique view. M = grossly dilated utricle (Müllerian duct cyst); straight arrows = bladder distended with air; curved arrow = coincident partial filling of left seminal vesicle and vas deferens. 34-year-old man with urgency, frequency, and suspected retrograde ejaculation. Lower left: Common urogenital sinus. Vagina (V) and urethra (U) join (at arrow) into a common urogenital sinus (S). Voiding cystourethrogram in a 3-week-old female pseudohermaphrodite with ambiguous genitalia and congenital adrenal hyperplasia. Lower right: Male pseudohermaphrodite. Bladder is distended with urine (black arrows). Retrograde urethrogram via hypospadiac meatus has fortuitously and selectively filled with contrast medium an extensive müllerian duct remnant consisting of vagina (V), cervix and cervical canal (C), and retroverted uterus (U). Residual contrast medium in hypoplastic anterior urethra (white arrow). 27-year-old man with small external genitalia, hypospadias, and perineal pain.
Vasoseminal vesiculography is most often used in the investigation of male sterility. The radiopaque contrast medium is introduced into the ductal system by direct injection into an ejaculatory duct following panendoscopy or, more commonly, by injection into the vas deferens after it has been surgically exposed through a small incision in the scrotal neck.
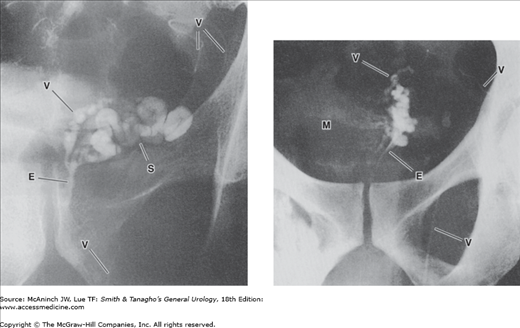
Figure 6–16.
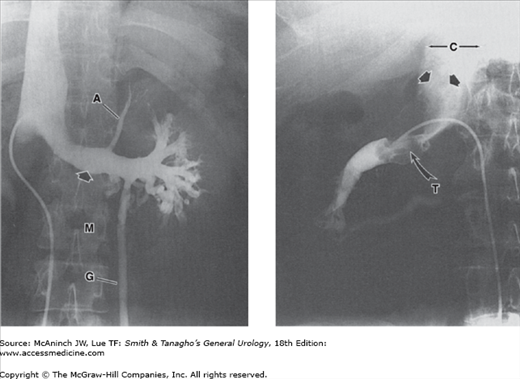
Vasoseminal vesiculography (vasography). Left: Normal left vasoseminal vesiculogram. V = vas deferens; S = seminal vesicle; E = ejaculatory duct. 40-year-old man with hypospermia. Right: Seminal vesiculitis. Bilateral vasogram. Mass (M) produced by the swollen, nonfilling right seminal vesicle has displaced both ejacula-tory ducts (E) toward the left and indented the medial aspect of the proximal left seminal vesicle and vas deferens (V). 33-year-old man with painful ejaculations after repair of right varicocele.
Lymphangiography has been largely abandoned and replaced by CT and MRI.
Nearly 50 years after Seldinger described techniques for percutaneous arteriography, catheter angiography maintains a limited role in treatment of some urologic disorders but is being replaced by CT or MRI for diagnostic examinations. Although an established imaging technique with proven value and an acceptable incidence of complications and morbidity, angiography is moderately invasive and relatively expensive.
Conventional arteriographic studies are performed almost exclusively by percutaneous needle puncture and catheterization of the common femoral arteries. Rapid sequence images are obtained during injection of nonionic contrast. Aortograms at the level of the renal vessels are followed by selective catheterization of renal arteries. CT and MR angiography involve peripheral injection of contrast media with breath hold rapid image acquisition through the targeted region of interest. CT angiography offers higher spatial resolution than magnetic resonance angiography (MRA), but carries the risks of radiation exposure and iodinated contrast usage.
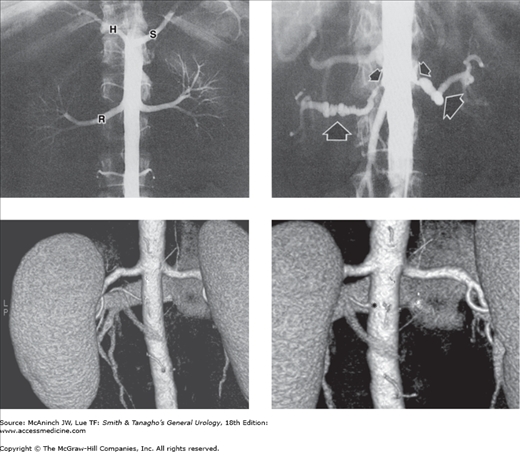
Figure 6–17.
Angiography: aortorenal arteriography. Upper left: Normal abdominal aortogram. The aortic catheter is hidden by the opacified normal aorta. Right (R) and left renal arteries and branches are well shown, as are the splenic (S) and hepatic (H) arteries arising from the celiac axis. The superior mesenteric artery is superimposed over the aortic silhouette and is not visible here. 28-year-old healthy female potential kidney donor. Upper right: Bilateral renal artery stenoses. Typical angiographic appearance and location of stenoses caused by atherosclerosis (small arrows) and fibromuscular dysplasia (large arrows). 58-year-old woman with abdominal bruits and a 16-year history of hypertension. Lower left: 3D coronal CT angiography image demonstrates an inferior accessory left renal artery (posterior view). Lower right: The left accessory renal artery origin (asterisk) is better demonstrated rotating the model in the axial plane. 65-year-old man undergoing preoperative evaluation for laparoscopic partial nephrectomy.
Indications for renal arteriography include suspected renal artery stenosis (renovascular hypertension), vascular malformations, tumor embolization to minimize surgical blood loss or treat bleeding tumors, and trauma. Diagnostic renal angiography to demonstrate renal vascular anatomy is uncommon, as this information may be obtained noninvasively. Complications from conventional catheter angiography include bleeding at the puncture site, contrast allergy or nephrotoxicity, and renal or distal emboli.
The common femoral veins, or less commonly the internal jugular veins, are catheterized for angiography of the inferior vena cava, renal, and adrenal veins. Venography is rarely used today since the information can be obtained at cross-sectional imaging (CT or MRI) in almost all cases. Adrenal and renal venography is performed occasionally for venous sampling to localize hormone secretion in patients with indeterminate noninvasive imaging studies.
Figure 6–18.
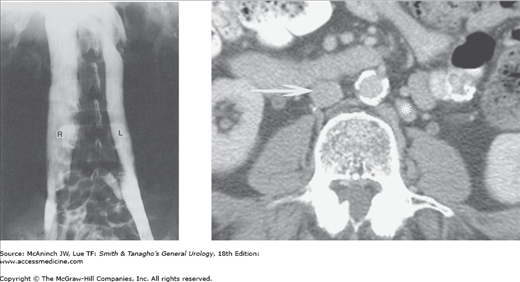
Angiography: inferior venacavography. Left: Double inferior vena cava (R, L). Persistent left supracardinal vein anomaly. 23-year-old man after orchiectomy for testicular teratocarcinoma. Right: Example of duplicated IVC on IV contrast enhanced axial CT. Normal IVC (arrow) and duplicated IVC (asterisk).
Figure 6–19.
Angiography: renal venography. Left: Normal left renal vein. On the left side, the adrenal (A) and gonadal (G) veins enter the renal vein (arrow
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree