Chapter 28 Radiologic hepatobiliary interventions
Radiologic Intervention
Radiologic intervention is applicable for a wide range of hepatobiliary diseases (see also Chapters 12, 19, 25, and 32). The use of these techniques in pancreatic disease is referred to in Chapter 25, Chapter 32, Chapter 54, Chapter 55A, Chapter 55B, Chapter 63A, Chapter 63B .
Treatment of Hepatic Cysts
Simple, nonparasitic hepatic cysts (see Chapters 69A and 69B) are the second most common benign lesions that involve the liver, following hemangiomas. They are found in approximately 1% to 2.5% of adults and occur more commonly in women and in the right liver. These cysts are lined with bile duct epithelium, contain clear fluid, and almost never communicate with the biliary tree (Forbes & Murray-Lyon, 1991). Simple cysts can be acquired from processes such as trauma or inflammation, or they may be congenital, such as in polycystic disease (see Chapter 69A, Chapter 69B ), with cysts possibly present in the kidneys and pancreas as well.
On cross-sectional imaging, the requisite feature of a simple hepatic cyst is a circumscribed, thin-walled, nonseptated lesion with lack of enhancement after intravenous contrast medium administration. Imaging modality–specific features include posterior acoustic enhancement on ultrasound (US), low attenuation on computed tomography (CT), and signal intensity similar to that of free water on magnetic resonance imaging (MRI). Wall thickening, the presence of septations, or lack of enhanced through-transmission on US should raise the question of another pathology, such as cystic metastasis, cystadenoma (see Chapter 79B), or hydatid disease (see Chapter 68), which must be thoroughly investigated (Mortele & Ros, 2001; Nisenbaum & Rowling, 1995). This evaluation is especially important before beginning treatment, and periodic imaging follow-up early in the course of therapy is prudent.
In the absence of symptoms or signs, simple liver cysts require no treatment. Traditional therapy of symptomatic cysts has been surgical, with laparoscopic deroofing currently favored by many surgeons (Gall et al, 2009; Mazza et al, 2009; see Chapter 65). Interventional radiologic techniques such as fine needle aspiration (FNA) and catheter drainage without or with cyst sclerosis have been used to treat simple cysts. With these techniques, symptomatic relief usually is achieved, although recurrence is common with aspiration alone.
Percutaneous chemical sclerosis of cysts with a variety of agents—including alcohol, tetracycline antibiotics, and povidone-iodine—either at the time of initial aspiration or after a period of catheter drainage usually provides successful therapy (vanSonnenberg et al, 1994). In the case of alcohol, it fixes the cells lining the cyst cavity, thereby preventing secretion of cyst fluid and subsequent enlargement (Bean & Rodan, 1985). Tikkakoski and colleagues (1996) described single-session treatment with alcohol for 59 symptomatic hepatic cysts in 25 patients. The cysts were evacuated via a 5-Fr drainage catheter, and absolute alcohol was instilled and left in for 20 minutes with the patient turned in various positions to maximize cyst wall exposure to the agent. In some cases, the procedure was repeated once or twice during the same session, after which the entire volume of injected ethanol was aspirated, and the catheter was removed.
Yoshida and colleagues (2003) successfully treated symptomatic solitary hepatic cysts with multiple minocycline injections and noted complete cyst regression without recurrence. Lopes and colleagues (1998) successfully treated seven patients with hepatic cysts by aspiration followed by instillation of tetracycline with a resultant decrease in all lesions. Kimura and colleagues (2005) performed repeated percutaneous instillations of alcohol after cyst aspiration and achieved subtotal or total cyst regression. Another study compared single-session alcohol sclerotherapy versus prolonged catheter drainage with negative pressure; they reported no differences in average volume reduction, final volume, and cyst regression (Zerem et al, 2008).
Laparoscopic cyst fenestration and deroofing are safe and are currently the most effective surgical approaches for symptomatic simple hepatic cysts. Image-guided (US or CT) hepatic cyst aspiration with chemical sclerosis using tetracycline or alcohol should be the treatment of choice for patients who are poor operative risks (Mazza et al, 2009).
Pyogenic Hepatic Abscess Drainage
Pyogenic hepatic abscesses result in considerable morbidity, and mortality occurs if abscesses are left untreated. Advances in cross-sectional imaging and interventional radiology have revolutionized the diagnosis and therapy of liver abscesses with a dramatic improvement in prognosis (see Chapter 66). Mortality has decreased from 65% (1952–1972) to 31% (1973–1993). This decrease occurred in the face of no significant change in patient characteristics, such as age and sex distribution, or incidence of associated conditions, such as diabetes mellitus, steroid use, chronic liver disease, chronic pancreatitis, and pyelonephritis (Huang et al, 1996). Another study from Denmark found the incidence rate of pyogenic abscesses increased from 6 to 18 per 1 million men and from 8 to 12 per 1 million women, and the 30-day mortality rate decreased from 40% for men and 50% for women to around 10% for both genders (Jepsen, 2005).
The liver is the most common site of abdominal visceral abscesses. Liver abscess is a result of either direct spread of infection from a contiguous focus, such as the biliary tree, or hematogenous seeding of the liver via the portal vein or hepatic artery. Biliary tract pathology is the most common cause of liver abscess. Of the nonbiliary diseases that lead to hepatic abscess, appendicitis with rupture and suppurative pylephlebitis, usually from infection in the female genital tract, are frequent causes (Zaleznik & Kasper, 1998).
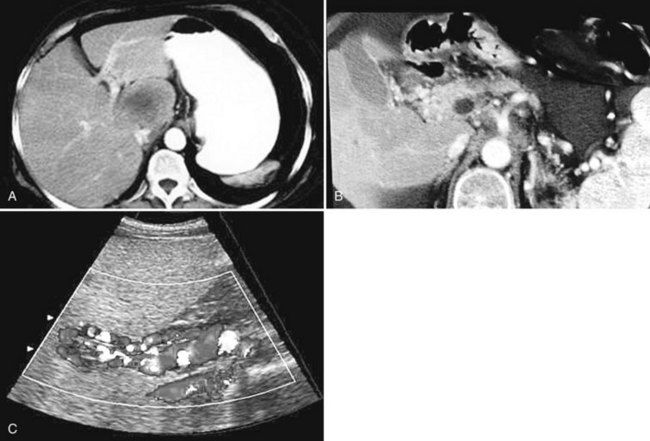
The constellation of a liver abscess and portal vein thrombosis with cavernous transformation of the portal vein may mimic the findings seen in hepatocellular carcinoma (HCC; Fig. 28.1). Clues to the correct diagnosis are the clinical and laboratory parameters that suggest infection and the absence of cirrhosis and portal hypertension, both of which are commonly seen with HCC. With successful treatment, complete resolution of both the abscess (see Fig. 28.1B) and the cavernous transformation of the portal vein (see Fig. 28.1C) is seen in many cases.
The microbial flora often reflect the source of the infectious process (see Chapter 11). In a study of 111 patients with pyogenic liver abscesses, Klebsiella species and Escherichia coli were the most common offenders (Lok et al, 2008). Anaerobes generally are encountered with a previous history of biliary intervention (e.g., surgery or stenting). With nonbiliary gastrointestinal (GI) sources of infection, polymicrobial gram-negative aerobes and anaerobes predominate, whereas systemic bacteremia is more likely to give rise to a single isolate, often S. aureus. Other reported microbes reflect individual patient factors, such as Entamoeba histolytica with a history of residence in or travel to endemic areas, and Candida species in neutropenic patients.
Clinical symptoms and signs are nonspecific. Of 111 patients with pyogenic liver abscess (Lok et al, 2008), 91% were seen initially with fever, 79% with right upper quadrant pain, 18% with anorexia, and 18% with septic shock. Laboratory abnormalities also are nonspecific. In the same study, 93% had hypoalbuminemia, 75% leukocytosis, 72% elevated alkaline phosphatase, and 59% elevated alanine transaminase.
US and CT are the imaging modalities of choice (Zaleznik & Kasper, 1997). Radionuclide scans (gallium, indium, and technetium) and MRI can provide further diagnostic information, but rarely are necessary.
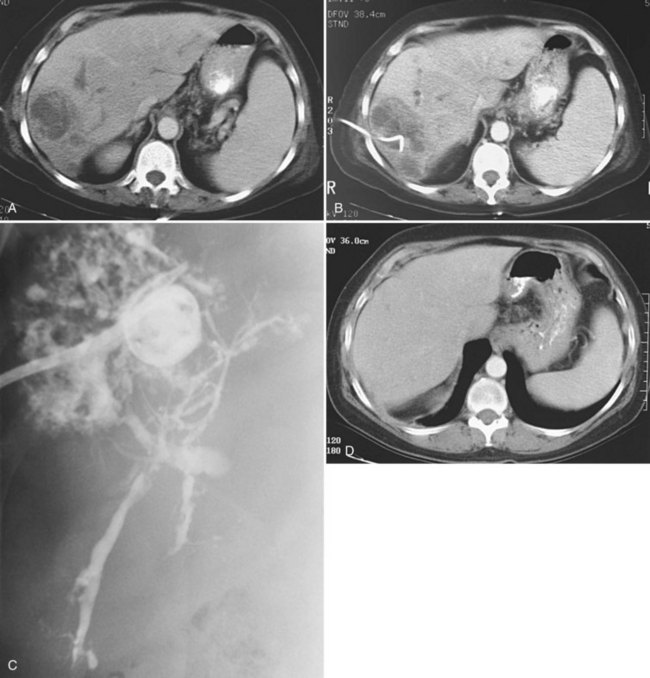
Uncomplicated pyogenic liver abscesses may be single or multiple, often are unilocular, and may be septated (Fig. 28.2). It may be impossible to evacuate complex multiloculated abscesses completely at the time of initial catheter placement (see Fig. 28.2B). Typically, there is free communication between the loculi (see Fig. 28.2C); consequently, most resolve completely when treated with a single catheter (see Fig. 28.2D) and appropriate antibiotics. If in doubt about adequate drainage of multiple locules or multiple abscesses, more than one catheter is indicated. Communication with the biliary tree may be seen when the drainage catheters are injected with contrast material as a part of performing an abscessogram (see Fig. 28.2C). The catheter contrast injections should not be performed until the pus has been evacuated to ensure that septicemia is not provoked iatrogenically.
Regarding the choice of percutaneous needle aspiration versus catheter drainage, opinions vary. Giorgio and colleagues (2006) described their experience with 118 patients treated with US-guided percutaneous needle aspiration and systemic antibiotic therapy. Cure was achieved in all 118 patients, and in 36 instances with only a single puncture. No recurrences were seen at 7 to 42 months of follow-up. The use of percutaneous needle aspiration and antibiotics is simple, less expensive, and more comfortable for the patient than catheter drainage. In practice, however, more than one aspiration is usually necessary for complete treatment with this strategy. Yu et al (2004) performed percutaneous needle aspiration in 64 patients with pyogenic liver abscess and concluded it was probably as effective as continuous percutaneous catheter drainage; however, their data were not statistically significant. Rajak and colleagues (1998) also compared the two methods of percutaneous treatment in 50 patients; they concluded that percutaneous catheter drainage was more effective than percutaneous needle aspiration. A failure of treatment was defined as a lack of response to a second attempt of percutaneous needle aspiration.
A more recent study (Zerem, 2007) also compared the two treatment methods in 60 patients, with the lack of response to percutaneous needle aspiration defined at three attempts. These authors agreed with Rajak and colleagues (1998) and concluded that percutaneous catheter drainage was more effective than percutaneous needle aspiration in the management of liver abscess. They opined that percutaneous catheter drainage is more efficient for multiloculated liver abscesses, and that percutaneous needle aspiration is a valid alternative for abscesses 5 cm or less in greatest diameter. Currently, treatment of pyogenic liver abscess with drainage catheter placement is the most commonly accepted method among interventional radiologists. Percutaneous aspiration without catheter drainage is not recommended except for occasional patients with one or more small liver abscesses that are too small for a catheter.
Surgical intervention for liver abscesses should be reserved for cases with coexisting surgically correctable causes of liver abscess (e.g., biliary tree abnormalities) or for cases of failed percutaneous therapy (see Chapter 66). A recent study demonstrated that partial liver resection is a safe and successful treatment for large, multiloculated complex pyogenic liver abscesses (Hope et al, 2008). Some associated biliary tree abnormalities may be amenable to endoscopic correction. In this subset of patients, endoscopic retrograde cholangiopancreatography (ERCP) might be preferred over surgery (Lam et al, 1999). Another study reported that previous endoscopic sphincterotomy was a good prognostic factor for early resolution of liver abscesses within 6 weeks (Wong et al, 2002).
Amebic Abscess
In a patient with a hepatic abscess and a history of travel to or residence in endemic areas, the possibility of infection with Entamoeba histolytica should be considered (see Chapter 67). Hepatic amebic abscess is the most common extraintestinal manifestation of amebiasis. Conditions that affect cell-mediated immunity—such as extremes of age, pregnancy, corticosteroid therapy, malignancy, HIV infection, and malnutrition—also may increase the chance that enteric E. histolytica infection results in extraluminal disease with liver involvement. The clinical findings are similar to the findings for pyogenic abscess; in addition, amebic abscesses may have superimposed bacterial infection, and they usually are solitary and most commonly located in the right lobe, adjacent to the liver capsule (Brindicci et al, 2006).
Serologic testing is the most widely used method to diagnose hepatic amebic abscess. Aspiration may be used to establish the diagnosis and to exclude secondary bacterial infection. When aspiration is used for diagnosis, the cavity should be aspirated as completely as possible. Amebic abscesses contain acellular, proteinaceous debris and an “anchovy paste,” a chocolate-colored fluid consisting predominantly of necrotic hepatocytes. Antigen tests and polymerase chain reaction on aspirated material may be helpful to confirm the diagnosis. Biopsy of the abscess wall may also establish the diagnosis if the other tests are inconclusive, which is the case in about 10% of patients (vanSonnenberg et al, 1985).
Amebic liver abscesses can generally be treated successfully with antibiotics alone (Chavez-Tapia et al, 2009). With clinical suspicion of amebic liver abscess, empiric treatment with metronidazole and a luminal agent (paromomycin or iodoquinol) may be started while awaiting confirmatory tests. Indications for catheter drainage of amebic abscess include failed medical therapy, secondary pyogenic infection, pregnancy, perforated abscess, false-negative serology, and left lobe abscess because of the proclivity to rupture or spread into the pericardium (vanSonnenberg et al, 1985; Ken et al, 1989; Hanna et al, 2000).
Echinococcal Disease
Echinococcal disease is an endemic zoonosis acquired from ingestion of Echinococcus granulosus eggs from infected animals (see Chapter 68). The most common location of hydatid cysts is the liver. Echinococcal cysts in the liver have a characteristic multiloculated or finely multiseptated appearance, and the right lobe is affected in 60% to 85% of cases.
Traditionally, surgery has been the recommended treatment for hepatic cysts (World Health Organization, 1996), but the combination of percutaneous drainage with drug therapy is a more recent alternative. Although percutaneous aspiration previously was contraindicated because of the risk of anaphylaxis, this complication has been shown to be rare (Akhan et al, 1996). Bret and associates (1988) demonstrated the safety of aspiration or drainage of these cysts in 13 patients, and none of the patients who underwent drainage was premedicated. If a catheter was placed for drainage, a scolicidal agent was injected into the cyst cavity after cystography. No complications or recurrences were reported 6 months to 1 year after treatment. Khuroo and colleagues (1997) compared percutaneous drainage with surgery for hepatic hydatid cysts and reported that percutaneous drainage, combined with albendazole, is an effective alternative to cystectomy. They demonstrated similar efficacy in cyst regression and disappearance, with advantages of a shorter hospital stay and a lower complication rate. Zerem and Jusufovic (2006) treated 72 patients with univesicular and multivesicular hepatic hydatid cysts with US-guided percutaneous drainage combined with albendazole, and 81% of the cysts in the univesicular group and 63% in the multivesicular group disappeared.
Percutaneous evacuation of cyst content (PEVAC) has been reported as a safe and effective treatment of multivesicular echinococcal cysts with or without cystobiliary fistulas, which contain undrainable material (Schipper et al, 2002). Hydatid cysts commonly have a communication with the biliary tree, which is a contraindication to injection of sclerosing agents, because this may cause diffuse injury to the biliary epithelium (Belghiti et al, 1986).
Transjugular Intrahepatic Portosystemic Shunt
The concept of transjugular hepatic intervention dates back to 1967, when Hanafee attempted diagnostic cholangiography via the transjugular intrahepatic route as a safer alternative to the percutaneous technique. At times, he inadvertently punctured portal vein radicles (Hanafee & Weiner, 1967). In 1969, Rosch and colleagues explored the transjugular route to visualize the portal venous system as an alternative to other available methods, such as splenoportography. The same year, Rösch and colleagues created the first transjugular intrahepatic portosystemic shunt (TIPS; see Chapter 76E) in dogs, using coaxial catheters to dilate the intrahepatic tract (Rösch et al, 1969, 1971). They discovered that without support, the tract would close; the plastic tubing used to “stent” the tract either thrombosed or migrated. Balloon dilation (Burgener & Gutierrez, 1979) and cryoprobes (Colapinto et al, 1983) were tried, but keeping the intrahepatic tract from shutting down remained a major problem. In the first human application of TIPS, Colapinto and associates (1982) used prolonged balloon inflation for 12 hours to keep the tract patent, but ultimately without success. The technique became a viable clinical option after refinement of metal stent technology in the late 1980s, when Richter and colleagues (1989) performed a landmark TIPS using a balloon-expandable Palmaz stent to maintain tract patency. The availability of Wallstents led to the rapid expansion and acceptance of the procedure.
Indications
The main indication for TIPS is the treatment of acute or recurrent variceal bleeding that has failed or is refractory to medical or endoscopic therapy, or both (see Chapter 75A, Chapter 75B, Chapter 75C, Chapter 76A, Chapter 76B, Chapter 76C, Chapter 76D, Chapter 76E ). This is especially true for patients who are bleeding from gastric or peristomal varices and those with severe portal hypertensive gastropathy. It is the preferred technique as a bridge to liver transplant, compared with surgical portosystemic shunts, because when performed correctly, TIPS does not alter extrahepatic vascular anatomy. It also is indicated for the control of refractory ascites and hepatic hydrothorax. TIPS may be used to treat Budd-Chiari syndrome in patients with moderate disease who fail to improve with anticoagulation (Boyer & Haskal, 2010).
Contraindications
There are several absolute and relative contraindications to TIPS placement. Absolute contraindications include congestive heart failure, sepsis, unrelieved biliary obstruction, severe pulmonary failure, and as the primary prevention of variceal bleeding (Boyer & Haskal, 2010). Relative contraindications to TIPS include the presence of HCC or hepatic metastases along the expected path of TIPS, hepatic vein obstruction, portal vein thrombosis, severe encephalopathy, polycystic disease of the liver, uncorrectable coagulopathy, and thrombocytopenia.
Technique (See Chapter 76E)
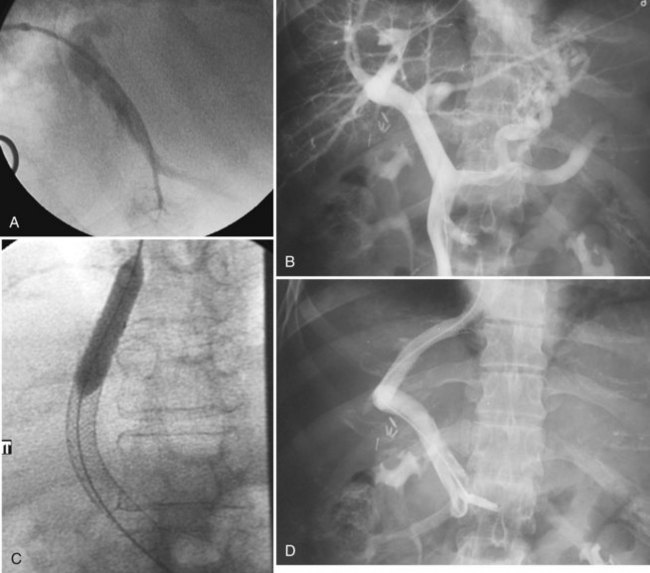
Various techniques and different systems are available for performing TIPS (Fig. 28.3). An imaging study using US, intravenous contrast-enhanced CT, or MRI to show a patent portal vein, hepatic veins, and hepatic venous anatomy is required. In an acutely bleeding patient, upper GI endoscopy confirms esophageal varices, excludes other sources of upper GI bleeding, and allows an attempt at endoscopic treatment. TIPS usually is performed under conscious sedation, but general anesthesia also may be used. Right internal jugular access is preferred, and a vascular sheath is placed. A selective catheter is advanced into one of the hepatic veins (see Fig. 28.3A).
The right hepatic vein is most commonly accessed for TIPS creation. Right atrial and free and wedged hepatic venous pressures are obtained, followed by hepatic and wedged hepatic venography. A specially designed needle is passed via the transjugular route from the right hepatic vein across the liver parenchyma into the right portal vein 2 to 3 cm from the main portal vein confluence. A guidewire is advanced into the superior mesenteric vein or splenic vein and is used to place a catheter for pressure measurements and venography (see Fig. 28.3B). The tract is predilated with a balloon (see Fig. 28.3C); an appropriately sized (8 to 12 mm) self-expanding bare stent or a specially designed covered stent is deployed, and the balloon is dilated to an appropriate size. Pressures are obtained, with the goal being a portal–systemic gradient of less than or equal to 12 mm Hg. If the gradient is greater than 12 mm Hg, certain stents (Wallstents) may be dilated with a 12-mm balloon. If this dilation does not result in a satisfactory pressure gradient, and collaterals fill persistently, or when stents other than Wallstent have been used, parallel TIPS should be considered. If the result is satisfactory (see Fig. 28.3D), the procedure is concluded, and baseline US studies are performed within the following few days.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree