Location
Onset
Diagnosis/management
Vessel
Gastroduodenal artery stump
Usually late
Angiography and embolization/stent
Hepatic artery
Late
Angiography and embolization
Inferior pancreaticoduodenal artery
Early
Reoperation following PD
Inferior pancreaticoduodenal artery
Late
Angiography and embolization/stent as this usually presents as a pseudoaneurysm
Splenic vein stump
Early
Reoperation following distal pancreatectomy
Splenic artery stump
Late
Angiography and embolization following distal pancreatectomy
Intrapancreatic arteries (smaller un-named)
Early or late
Early—reoperation
Late—angiography and embolization
Anastomoses
Hepaticojejunostomy
Early
Reoperation
Pancreaticojejunostomy
Early
Reoperation
Gastrojejunostomy
Early or Late
Endoscopy or reoperation
Early PPH (within 24 h after surgery) is most commonly the result of technical failure to properly secure the inferior pancreaticoduodenal arteries (IPDAs). One can also see bleeding at any of the three anastomotic suture lines (following PD) and rarely a GDA stump hemorrhage due to failure to properly secure this vessel. If the SMA dissection is performed sharply with direct identification and ligation of the IPDAs at their origin from the SMA, this complication can largely be avoided. Intra-abdominal hemorrhage from poorly secured IPDAs would present as early postoperative intra-abdominal hemorrhage and would require immediate reoperation. Bleeding from the post-PD reconstruction (pancreatic, biliary, or gastric anastomosis) is very uncommon, and the anastomosis of greatest risk is the pancreaticojejunostomy if an invagination anastomosis is performed. With this type of anastomosis, the cut surface of the pancreas is open to the inside of the jejunum and small vessels which are partially cauterized may retract at the time of pancreatic transection only to bleed when the patient is in the recovery room or during the first postoperative night. Hemorrhage from the biliary anastomosis should not occur, and bleeding from the gastrojejunostomy is also very uncommon in the absence of a technical error. Marginal ulceration at the gastrojejunostomy, if it were to occur, presents months or years after the date of surgery. Yekebas et al. presented an analysis of 1669 consecutive pancreatic resections and in their experience, early PPH was due to 3 causes: (1) technical failures in terms of inadequate hemostasis in the operative field always associated with extraluminal PPH (IPDAs being the most common involved vessels); (2) suture line of gastroenteric or one of the enteroenteric anastomoses leading uniformly to intraluminal PPH on the first or second postoperative day; and (3) resection cavity or transection surface of the pancreas resulting in PPH originating from the pancreatico-enteric anastomosis [15].
Late PPH may occur from a gastrointestinal source but more commonly originates from an intra-abdominal site often associated with intra-abdominal infection or abscess formation due to leakage of an anastomosis (most commonly the pancreaticojejunostomy). Intra-abdominal infection is thought to be the major cause of late PPH due to erosion into ligated vessels, most notably the GDA. Bleeding from a disrupted anastomotic suture line can also be caused by intra-abdominal infection and can mimic bleeding from major vessels [7, 8, 16]. Finally, some patients may present with bleeding from the wound after a wound infection but significant hemorrhage from this etiology is uncommon.
The core difference between the etiology of early and late PPH is the association of late PPH with pancreatic fistula and intra-abdominal infection. This finding is consistent throughout the surgical literature which notes an elevated risk of late PPH in patients with pancreatic fistula as well as a near 100 % prevalence of pancreatic fistula in patients who exhibit late arterial bleeding [16, 17]. Surgical reports are consistent in describing a sequence of events at the beginning of which pancreatic fistula causes erosions, pseudoaneurysms, and other vascular irregularities, which eventually result in clinically significant hemorrhage. Clearly, the majority of postoperative pancreatic fistulas do not result in late PPH and the cause of PPH within the population of patients who have a pancreatic leak is likely multifactorial. Extended lymphadenectomy or the need for concomitant adjacent organ resection (resulting in a large retroperitoneal space), soft texture of the pancreatic remnant in the setting of a complete anastomotic disruption, or insufficient drainage of pancreatic fistula (failure to obtain source control) may be the cofactors increasing the risk of fistula-induced vascular injury and PPH [16–18].
Possible pathophysiologic explanations for pancreatic anastomotic leak-associated late PPH include enzymatic digestion of the blood vessel wall by trypsin, elastase, and other pancreatic exocrine enzymes, intra-abdominal infection/abscess with direct involvement of the vessel wall, and/or vascular injury at the time of operation that leads to pseudoaneurysm formation [3]. Most reports and anecdotal clinical observations favor the theory of local sepsis resulting from pancreatic fistula as the main cause of late PPH. Local sepsis may erode the vascular wall and adjacent bowel. This mechanism of injury may result in acute arterial bleeding with or without arterial pseudoaneurysm formation, which typically occurs days to weeks after the operation [19]. There is minimal data regarding the impact of newer energy devices, especially when using them for ligating the IPDAs arising from the SMA; however, anecdotal experiences with such situations have generated reason for caution. Many of us have managed PPH in patients where the use of such energy devices close to arterial structures has been implicated in the etiology of late PPH.
Skeletonization of the hepatic artery and SMA which is performed with PD, and similar dissection of the celiac artery and splenic artery stump associated with distal pancreatectomy make these vessels vulnerable to pseudoaneurysm formation due to local sepsis arising from the pancreatic fistula, anastomotic leakage, or intra-abdominal abscess [20]. In a series reported by Lee et al., of 27 patients with PPH, 26 had an antecedent pancreatic fistula, as shown by drain amylase level and computed tomography (CT) findings. This report confirms the association between late PPH and pancreatic fistula. The onset of the infectious complication ranged from 7 to 13 days but the hemorrhage developed after postoperative day 28 in 9 patients. The high frequency of late-onset (after 4 weeks from the date of operation) hemorrhage in this study led the authors to conclude that PPH can occur more than 4 weeks postoperatively, particularly in patients with pancreatic fistula and/or a complicated initial postoperative course [21].
Prevention of Late PPH
The Falciform Ligament
When opening the abdomen, we carefully preserve the falciform ligament (obliterated umbilical vein) for later use as coverage of the GDA stump, vascular anastomoses, or other peripancreatic vessels [22]. A pedicled falciform ligament is easily and rapidly obtained during a midline abdominal incision. After incising the linea alba, the preperitoneal fat is dissected laterally (to the left) when incising the peritoneum. The falciform ligament is mobilized by dividing it near the umbilicus and incising its anterior peritoneal reflections along the posterior rectus sheath. An additional length is obtained by continuing the anterior incision cephalad to the anterior surface of the liver. The pedicled falciform ligament is completed by taking down the attachments of the liver until just the obliterated umbilical vein remains attached. Note that the pedicled falciform ligament (Fig. 26.1) normally reaches the space between the pancreaticojejunostomy and the major vessels exposed during resection. After completion of the pancreatectomy, the pedicled falciform ligament is spread widely anterior to the common/proper hepatic artery with special attention to coverage of the GDA stump. A robust flap usually also covers the superior mesenteric vein (SMV), portal vein (PV), and splenic vein confluence effectively separating the vessels from the afferent jejunal limb (Fig. 26.2). When a distal pancreatectomy is performed, the pedicled falciform ligament can be fixed with 4-0 prolene sutures to the remnant pancreas thereby reinforcing the pancreatic closure. This procedure enables the complete separation of these vessels from the pancreas in the event that a pancreatic fistula and associated abscess were to develop.
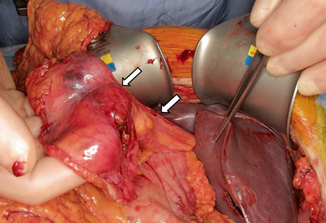
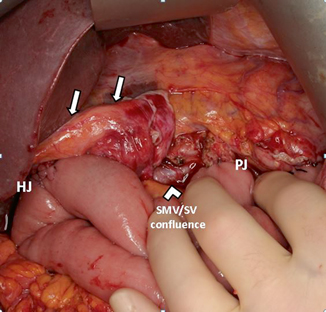

Fig. 26.1
Intraoperative photograph of preserved falciform ligament pedicle flap. Debakey forceps are retracting the liver. White arrows point to the falciform flap

Fig. 26.2
Intraoperative photograph of completed pancreaticoduodenectomy. The falciform ligament pedicle flap ( white arrows) completely covers the common hepatic artery and GDA stump from any possible PJ leak. HJ hepaticojejunostomy, PJ pancreaticojejunostomy, SMV superior mesenteric vein, SV splenic vein
The Portal Dissection
The portal dissection is initiated by removing the lymph node that lies directly anterior to the common hepatic artery (CHA) proximal to the right gastric artery and GDA. This facilitates exposure of the CHA proximal and distal to the GDA. The right gastric artery is ligated and divided followed by the GDA. Dissection of the hepatic artery should be performed with gentle, sharp dissection, especially in patients who have received prior chemotherapy or chemoradiation and in those with extensive peritumoral inflammation from a previous laparotomy or stent-related pancreatitis. Blunt dissection at the GDA origin can result in intimal dissection of the hepatic artery. Division of the GDA allows mobilization of the hepatic artery and exposure of the anterior surface of the PV directly posterior to the inferior border of the CHA. The PV should always be exposed in this way before dividing the common hepatic duct. Care during this critical step in the performance of PD can minimize trauma to the hepatic artery and allow for a secure closure of the GDA stump [22].
GDA Ligation
Occasionally, ligation of the GDA is complicated by close proximity of the pancreatic tumor. If the tumor extends to within a few millimeters of the GDA, our technique is to obtain proximal and distal control of the hepatic artery and then divide the GDA flush at its origin. The resulting arteriotomy can be closed primarily with interrupted 6-0 prolene sutures. If 2 mms of GDA origin is available, we often use a small vascular pledget, as the hepatic artery can be quite fragile in this location; if the arteriotomy is flush with the CHA, a pledget cannot be used. When the tumor extends to the GDA origin, we divide the GDA prior to any form of ligation of the distal GDA on the specimen side. The GDA on the specimen side is suture ligated with 4-0 Prolene after it is divided; control of back-bleeding from this vessel is easily accomplished with simple hand pressure if a complete Kocher maneuver was performed earlier in the operation. This maneuver decreases trauma and handling of the GDA and decreases chances of intimal dissection of the hepatic artery [22]. When adequate length of GDA allows for a simple ligation, we usually use a 0-silk tie on the hepatic artery side with a 4-0 Prolene suture on the specimen side (so as to avoid unnecessary mobilization which is often needed to place a tie distally on the specimen side).
Reinforcing the Pancreatic Transection Site (Distal Pancreatectomy)
When performing a distal or subtotal pancreatectomy, the remnant pancreas can lead to a potential pancreatic fistula and subsequent PPH from a splenic artery pseudoaneurysm. We routinely divide the pancreas and perform the pancreatic closure either with Gore-Tex reinforced staples or with pledgeted sutures. The limitation to using the stapler is in proximal neck/body tumors where there is limited room (due to the proximity of the intrapancreatic bile duct) for achieving an adequate margin. In addition, as one moves to the patient’s right of the pancreatic neck (and enters the region of the pancreatic head), the pancreas becomes too thick for a staple line. In this scenario, after confirming a negative margin, we identify the pancreatic duct and close it directly with a horizontal mattress suture. We then close the remaining pancreas with additional horizontal mattress sutures with a pledget on both the posterior and anterior surfaces (Fig. 26.3). The first such pledgeted suture is placed at the site of the pancreatic duct so that the duct closure is covered by the location of the pledget. Both a stapled closure and a suture closure with pledgets are done to minimize the risk of pancreatic fistula, which can increase the risk of PPH.
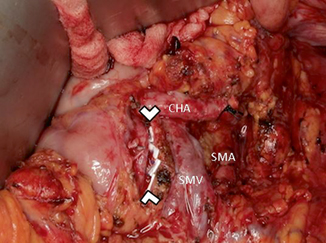





Fig. 26.3
Intraoperative photograph of a completed distal pancreatectomy. Arrowheads point to the cut margin of the pancreas closed with pledgeted sutures. CHA common hepatic artery, SMA superior mesenteric artery, SMV superior mesenteric vein
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








