Chapter 25 Postoperative complications requiring intervention, diagnosis, and management
Overview
The literature regarding postoperative adverse events is difficult to interpret, because most reports are retrospective in nature, and the definitions of adverse events vary among institutions. Complication rates may vary by how hard one looks and by what definition an individual surgeon uses to declare a postoperative event as a complication rather than normal postoperative recovery. This is particularly true for retrospective analyses, which likely overlook many events. Over the past decade these difficulties have been addressed through the development of more carefully defined systems for documenting and grading perioperative complications to improve reporting and provide more uniform methods for comparing outcomes across institutions and countries (Bassi et al, 2005a; Clavien et al, 2009; DeOliveira et al, 2006; Grobmyer et al, 2007; Vin et al, 2008). These systems of standardized grading and reporting of adverse events have become the norm in medical oncology trials and are becoming so in prospective surgical trials. Such standardization should improve our ability to define the morbidity of a procedure and to compare morbidity among approaches and institutions.
Over the last 15 years, major hepatic and pancreatic resections have been performed with increasing frequency (Allen, 2007; Brennan et al, 2004; Cameron et al, 2006; Jarnagin et al, 2002; Nguyen et al, 2009; Riediger et al, 2007; Balcom et al, 2001). This increase has been most apparent at high-volume institutions and may be secondary to a trend in specialization. Multiple reports have shown a link between high-volume surgeons and institutions and decreased postoperative mortality for both pancreatic and liver resection (Begg et al, 1998; Birkmeyer et al, 2003; Eppsteiner et al, 2008, 2009; Lieberman et al, 1995). Despite the observed decrease in postoperative mortality, morbidity remains statistically unchanged over time with most large series reporting a 35% to 45% major complication rate following either pancreatic or hepatic resection (Ho et al, 2005; Simons et al, 2009; Turaga et al, 2008).
This apparent improvement in postoperative mortality without an observed decrease in morbidity suggests an improved ability to successfully rescue patients who experience significant postoperative complications. Management of serious complications following pancreatectomy and liver resection has shifted from operative reexploration to interventional or endoscopic procedures (Vin et al, 2008). High-quality imaging and increasing familiarity with the specific perioperative events has led to earlier detection and intervention and a subsequent reduction in the need for reoperation. Percutaneous drainage procedures are now the typical approach to postpancreatectomy leak or abscess, and they can be performed with minimal sedation and low morbidity.
Pancreatectomy
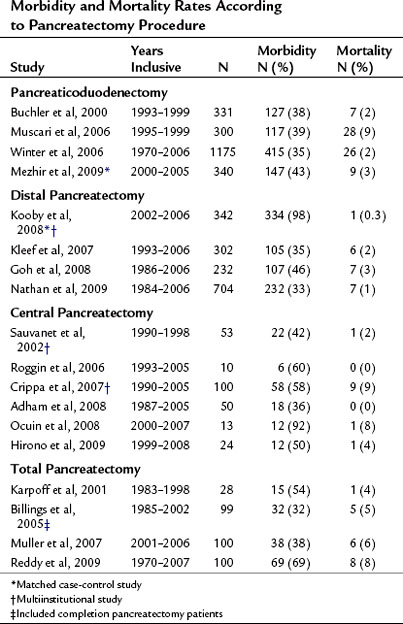
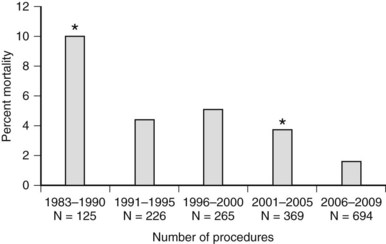
The mortality associated with pancreatectomy has significantly improved over the last 20 years; however, the morbidity has remained essentially unchanged. Operative mortality following pancreatic resection in large series reported since 2000 is presented in Table 25.1. The overall perioperative mortality has ranged from 2% to 9% for pancreaticoduodenectomy (PD), and reported morbidity rates are between 35% and 43% (Buchler et al, 2000; Mezhir et al, 2009, Muscari et al, 2006; Winter et al, 2006a). Recent studies show significantly improved mortality rates compared with reports from the 1980s and 1990s. This trend is illustrated in perioperative mortality data over a span of more than 25 years at Memorial Sloan-Kettering Cancer Center (MSKCC; Fig. 25.1). In a 5-year period between 1983 and 1990, the mortality rate was 10% in 125 pancreatic resections; between 2006 and 2009, the operative mortality rate was 1.7% in 694 pancreatic resections. The operative mortality rates presented in Table 25.1 are remarkably similar despite the fact that these data are from different institutions around the world.
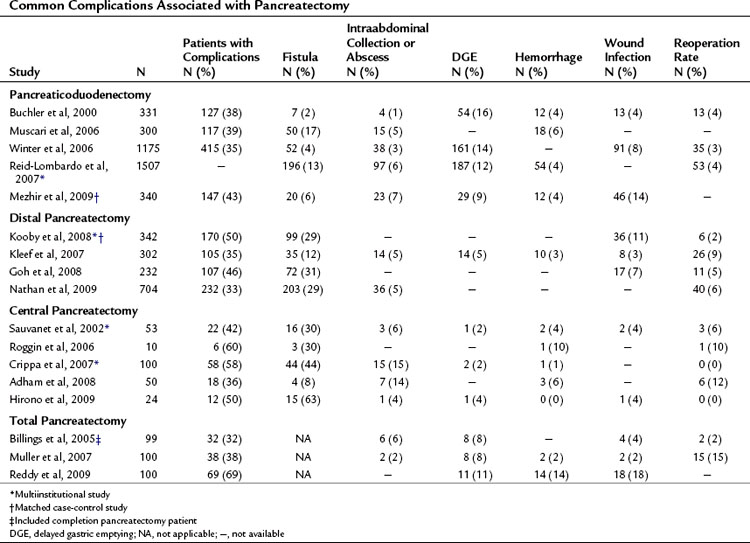
Complications may occur at the operative site (hemorrhage, leak, obstruction) or remotely (deep venous thrombosis, cardiac arrhythmia, pneumonia). Despite efforts to optimally manage comorbid conditions preoperatively, cardiac and pulmonary events occur following major surgeries. In addition, the combination of prolonged operative time, fluid volume, and underlying malignancy may increase the risk of certain complications unrelated to technique, such as pulmonary embolus, atrial dysrhythmia, and pneumonia. The most common severe complications following pancreatic and hepatic resection are directly related to the technical aspects of the operation itself. The frequency of the most commonly reported major complications following pancreatectomy are listed in Table 25.2. These include delayed gastric emptying (DGE), intraabdominal fluid collection or abscess, hemorrhage, wound infection, and anastomotic fistula. A comprehensive list of complications taken from a series of 204 consecutive pancreaticoduodenectomies at MSKCC from 2001 to 2003 is presented in Table 25.3 (Grobmyer et al, 2007).
Table 25.3 Complications After Pancreaticoduodenectomy
| Complication | N | % |
|---|---|---|
| Anastomotic leak, pancreas | 24 | 12 |
| Wound infection | 22 | 11 |
| Delayed gastric emptying | 14 | 7 |
| Hemorrhage | 10 | 5 |
| Intraabdominal abscess | 8 | 4 |
| Fascial dehiscence or evisceration | 7 | 3 |
| Supraventricular arrhythmia | 7 | 3 |
| Urinary tract infection | 6 | 3 |
| Anastomotic leak, biliary | 6 | 3 |
| Hypotension, shock | 5 | 2 |
| Cellulitis | 3 | 1.5 |
| Clostridium difficile colitis | 3 | 1.5 |
| Congestive heart failure, left ventricular dysfunction | 3 | 1.5 |
| Myocardial infarction | 3 | 1.5 |
| Renal failure | 3 | 1.5 |
| Apnea or hypoxia | 2 | 1 |
| Atelectasis | 2 | 1 |
| Catheter-related infection | 2 | 1 |
| Deep venous thrombosis | 2 | 1 |
| Dehydration | 2 | 1 |
| Anastomotic leak, intestinal | 2 | 1 |
| Gastrointestinal bleeding | 2 | 1 |
| Pleural effusion | 2 | 1 |
| Pneumonitis | 2 | 1 |
| Sepsis | 2 | 1 |
| Small bowel obstruction | 1 | 0.5 |
| Angina, cardiac ischemia | 1 | 0.5 |
| Aspiration | 1 | 0.5 |
Modified from Grobmyer SR, et al, 2007: Defining morbidity after pancreaticoduodenectomy: use of a prospective complication grading system. J Am Coll Surg 204(3):356-364.
Pancreatic leak/fistula continues to be one of the most common and most difficult complications following pancreatectomy. Multiple studies have reported pancreatic fistula to be more common following central or distal pancreatectomy compared with pancreaticoduodenectomy (Pratt et al, 2006; Goh et al, 2008; Kleeff et al, 2007; Kooby et al, 2008; Nathan et al, 2009; Ferrone et al, 2008; Reid-Lombardo et al, 2007). The series reported in Table 25.1 also demonstrate that central pancreatectomy (Adham et al, 2008; Crippa et al, 2007; Hirono et al, 2009; Ocuin et al, 2008; Roggin et al, 2006; Sauvanet et al, 2002) and total pancreatectomy (Billings et al, 2005; Karpoff et al, 2001; Muller et al, 2007; Reddy et al, 2009) are procedures associated with slightly higher overall morbidity than PD and distal pancreatectomy, and total pancreatectomy is associated with a slightly higher mortality rate than PD.
Defining and Grading Complications After Pancreatectomy
Accurate identification and reporting of perioperative complications are vital to improve quality of care and to compare outcomes among different institutions and time periods. Without a standardized system for complication recording, there is a significant risk of inaccuracy and underreporting. Recently, several institutions have implemented specific classification and grading systems for surgical complications (Clavien et al, 1992, 2009; DeOliveira et al, 2006; Grobmyer et al, 2007). These systems have developed simplified definitions of postoperative complications and a grading system for severity using therapy-oriented grading schema.
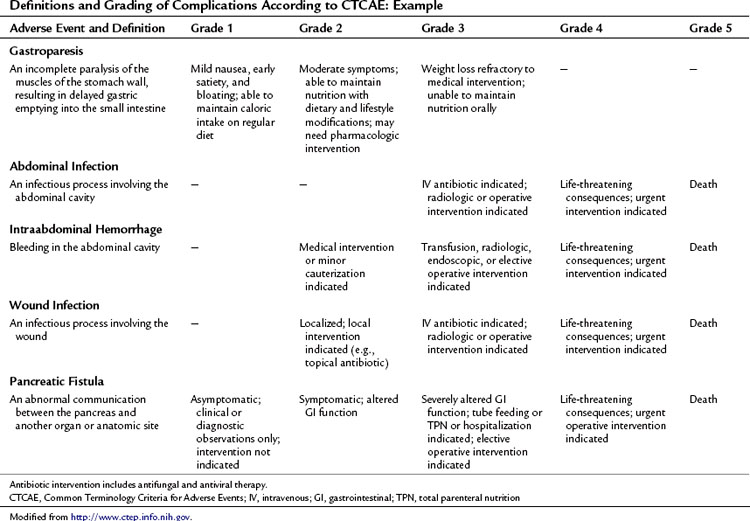
This approach to defining and grading adverse events has been well established in medical oncology. The Common Terminology Criteria for Adverse Events (CTCAE) developed by the National Institutes of Health Cancer Therapy Evaluation Program (CTEP) has been utilized in clinical trials since 1982 (National Cancer Institute’s Cancer Therapy Evaluation Program). CTCAE is an example of a universally employed terminology system for complication reporting that has been utilized across medical disciplines. This system also includes a limited schema for postoperative complications. Table 25.4 demonstrates an example of the grading system for common complications following pancreatectomy. The grade of the complication increases with the invasiveness of the treatment required.
Among the complications listed, pancreatic fistula is one of the most severe that can occur after pancreatectomy. The reporting of this complication is arguably the most problematic, as definitions have varied. Pancreatic fistula often precedes other complications, such as abscess and hemorrhage. Inherent to its cause, complications from pancreatic leak, or any anastomotic leak for that matter, often overlap (see Management of Fistula, Leak, and Abscess below). When a postoperative patient has fever, tachycardia, and leukocytosis and cross-sectional imaging reveals a perianastomotic fluid collection, the etiology of this collection is likely an anastomotic leak. When the collection is drained, if it is infected, it may be recorded or defined as an abscess. If the fluid is amylase rich, one investigator may report it as a pancreatic fistula and another may report it as a pancreatic leak. This ambiguity is reflected in reports that reflect rates of intraabdominal abscess for pancreatectomy ranging from 1% to 15%. Moreover, the incidence of fistula ranges from 2% to 27% for PD and 12% to 31% for distal pancreatectomy in part as a result of the inconsistent definitions utilized in studies from different institutions.
Toward a Unifying System for Reporting Pancreatic Fistula
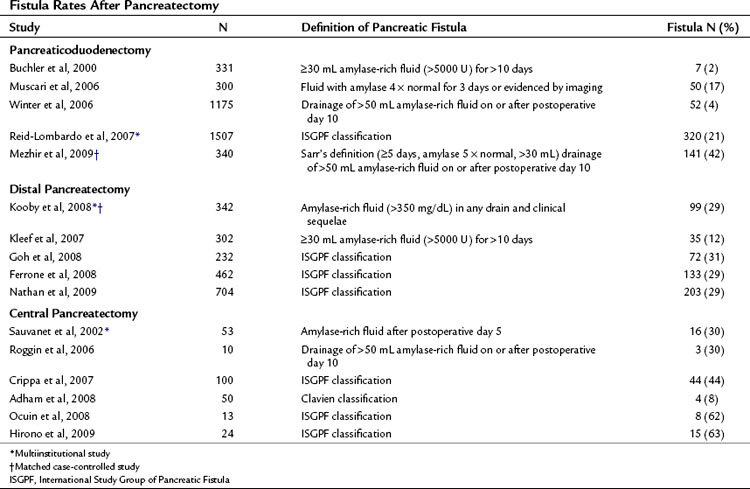
Pancreatic fistula has been recently defined by the International Study Group of Pancreatic Fistula (ISGPF; Bassi et al, 2005a). This system has served as a foundation for defining and reporting pancreatic fistula among numerous studies and is an example of the efforts to unify complication reporting. The study group comprised an international panel of pancreatic surgeons working in high-volume centers, and it aimed to develop a simplified and universal definition of pancreatic fistula with a grading system based on clinical impact. Fistula was defined as “drain output of any measurable volume of fluid on or after postoperative day 3 with amylase content greater than three times that of normal serum amylase.” Three grades were applied according to clinical impact, from grade A (none) to grade C (significant; Table 25.5). As demonstrated in Table 25.6, many institutions have implemented this system for reporting the rates and outcomes of pancreatic fistula.
Independent prospective validation of the ISGPF definition and grading system has been reported. Pratt and others (2006) prospectively analyzed postoperative complications in 176 consecutive patients after pancreaticoduodenectomy. There were 53 patients with confirmed fistulae (30% incidence), categorized in the various grades: 26 patients (15%) were grade A, 21 (12%) were grade B, and 6 (3%) were grade C. No deaths were related to fistula in this study. Patients with grade A fistulas had shorter hospital stays and fewer secondary complications than patients with grade B and C fistulas, although the overall rate of secondary complications between grade B and C fistulas was not significantly different (76% vs. 100%, respectively, P = .2). Patients with grade C fistulas had a higher frequency of intensive care unit (ICU) transfer and blood transfusions and longer hospital stays compared with those who had grade B fistulas, and five of the six patients with a grade C fistula were discharged to a rehabilitation facility, significantly more than patients with grade A and B fistulae.
Randomized Controlled Trials Aimed at Reducing Complications
Multiple prospective randomized controlled trials have been performed to evaluate technique and complications following pancreatic resection. Selected prospective trials published since 2000 are presented in Table 25.7. In general, these studies have reported conflicting outcomes with no specific technique demonstrating superiority. The interpretation of surgical technique trials is difficult, as most trials randomize patients between “the standard technique” at a given institution and a “new,” perhaps less familiar technique. These studies are often underpowered, and it is therefore not surprising that equivalency is concluded. Fortunately, none of the reported techniques have been shown to be inferior from an oncologic standpoint, and therefore any of these reported techniques may be considered acceptable in the appropriately selected patient.
Table 25.7 Prospective Randomized, Controlled Trials Aimed at Reducing Perioperative Complications After Pancreatectomy
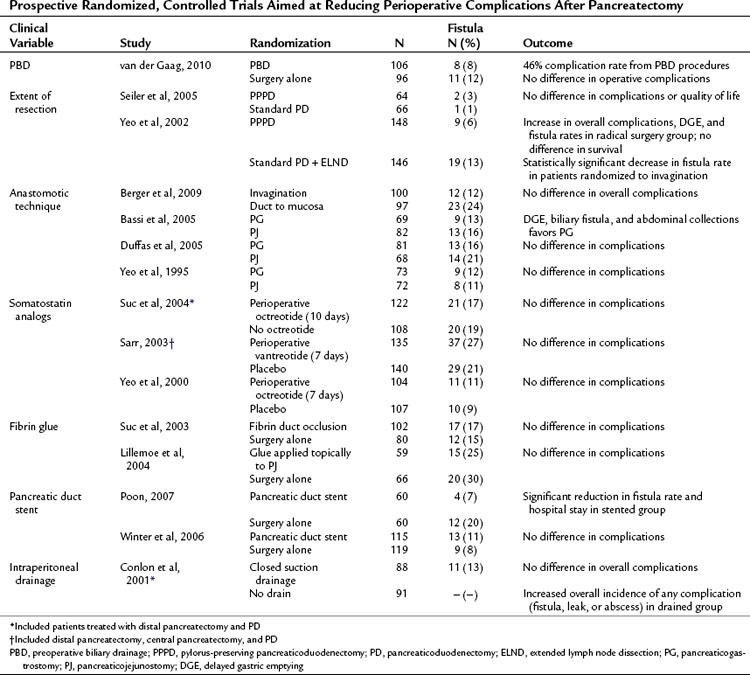
Routine preoperative biliary drainage (PBD) has been evaluated in multiple prospective randomized trials. These trials have evaluated both percutaneous external and endoscopic internal drainage (see Chapters 27 and 28). These studies, as well as multiple retrospective case-matched reports, have documented an increase in perioperative complications in patients undergoing PBD (Mezhir et al, 2009; Povoski et al, 1999). A recent prospective, controlled clinical trial randomized patients with serum bilirubin levels between 2.3 and 14.6 mg/dL to PBD or no PBD prior to pancreaticoduodenectomy (van der Gaag et al, 2010). The authors reported 39% significant complications in the no PBD group compared with 74% in the PBD group (P < .001). These data support previous prospective and retrospective studies and do not support routine PBD in patients with moderately elevated bilirubin.
Pylorus preservation has been evaluated as a technique to decrease the morbidity of PD (see Chapter 62A). Standard PD with partial gastrectomy versus pylorus-preserving PD (PPPD), with or without extended lymphadenectomy, has also been investigated in randomized trials. As demonstrated in Table 25.7, no benefit was demonstrated for either approach in regard to operative or long-term oncologic outcomes, but extended lymphadenectomy was associated with increased perioperative morbidity, including DGE and pancreatic fistula (Yeo et al, 2002; Seiler et al, 2005).
Pancreatic fistula rates have been assessed according to anastomotic technique in an attempt to reduce postoperative pancreatic fistula. Randomized comparisons have been made between pancreaticojejunal invagination to direct duct-mucosa anastomosis and pancreaticojejunostomy (PJ) to pancreaticogastrostomy (PG). Berger and colleagues (2009) performed a two-institution randomized controlled trial comparing pancreatic invagination to duct–mucosa anastomosis with patients stratified based on the texture of the pancreas. A significant reduction in fistula rates were seen when using the invagination technique (12%) compared with duct-mucosa anastomosis (24%), and leak rates were significantly higher overall in soft glands. Bassi and colleagues (2005b) showed a decrease in DGE, biliary fistula, and abdominal collections with PG; however, these findings have not been replicated by other groups (Duffas et al, 2005; Yeo et al, 1995). No study to date has demonstrated convincingly an optimal method for pancreatic stump closure following distal pancreatectomy (Knaebel et al, 2005); the results of an ongoing, large, multiinstitutional randomized trial may provide some guidance (Diener et al, 2008).
Other adjuncts to reduce pancreatic leak have included somatostatin analogs, stents, and tissue glues. The perioperative use of somatostatin analogs was found to reduce fistula rates in several European trials (Montorsi et al, 1995; Buchler et al, 1992; Friess et al, 1995). However, these findings have not been reproduced in recent large, single, and multiinstitutional studies performed in the United States (Sarr, 2003; Suc et al, 2004; Yeo et al, 2000). Fibrin glue has been applied in two settings to determine its role for reducing pancreatic fistula, applied in a perianastomotic fashion (Lillemoe et al, 2004) or as a plug into the pancreatic duct prior to anastomosis (Suc et al, 2003). Neither study showed a reduction in fistula rates. Two prospective trials evaluated pancreatic stent placement at the time of anastomosis; in one study, a pancreatic duct stent significantly reduced the rate of pancreatic fistula (Poon et al, 2007), but the other study did not demonstrate any difference in fistula rates or overall complications (Winter et al, 2006b).
The routine use of intraperitoneal drains following elective pancreatic surgery remains an area of debate. Conlon and colleagues (2001) randomized 179 patients after pancreatic resection (PD or distal pancreatectomy) to placement of intraperitoneal drains or no drains, demonstrating no benefit to routine drainage. Moreover, patients in the drainage group were more likely to have an intraabdominal abscess, collection, or fistula.
Management of Postpancreatectomy Complications
Pancreatic Leak, Fistula, and Abscess
Vin and colleagues (2008) reported a series of 908 pancreatic resections and the rate and management of pancreatic fistula, leak, and abscess. As demonstrated in Figure 25.2, a significant overlap was found among these three separately defined complications in the 158 patients in which they occurred. This further highlights the difficulty in defining discrete complications, the crossover between different definitions, and the clinical relevance of complication grading. In this study, a pancreatic fistula, defined as clinical signs or symptoms with amylase-rich drainage greater than 50 mL/day beyond postoperative day 10, was identified in 92 patients; however, in 63 of these (69%), the fluid that was drained was culture positive. These patients could therefore have been defined as having either or both a pancreatic fistula and postoperative abscess. Regardless of the definition, patients who develop a pancreatic fistula, leak, or abscess will often evidence similar findings and be treated in a similar fashion. Importantly, the type of complication (fistula, leak, or abscess) was not associated with presentation (fever, leukocytosis) or overall morbidity (duration of drainage, length of stay, ICU admission); however, these factors were similar in patients who had a similar grade of complication.
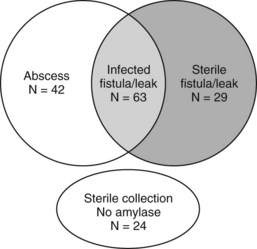
(Modified from Vin Y, et al, 2008: Management and outcomes of postpancreatectomy fistula, leak, and abscess: results of 908 patients resected at a single institution between 2000 and 2005. J Am Coll Surg 207[4]:490-498.)
Vin and colleagues (2008) reported a 90-day mortality rate of 5% in patients who developed fistula, leak, or abscess. These complications were more common in men and in patients undergoing treatment for bile duct, ampullary, and duodenal tumors (63 [31%] of 206) compared with pancreatic tumors (95 [14%] of 702; P < .001). Type of resection (PD vs. distal pancreatectomy), tumor histopathologic subtype, and PBD were not associated with occurrence of fistula, leak, or abscess. Surgical drains were placed in 88 patients with these complications. The surgical drain alone, without additional interventional radiologic management, was successful for treatment in 16 (18%) of 88 patients, and 133 patients were managed with percutaneous drainage alone.
Delayed Gastric Emptying
Delayed gastric emptying (DGE) after PD is common (see Table 25.2) and is typically reported in 9% to 16% of patients. A review of randomized controlled trials for treatment of DGE has been recently published (Traverso & Hashimoro, 2008), examining the literature for level I evidence, and four trials were identified that focused on the prevention of DGE. The largest study from Yeo and others (1993) randomized patients to erythromycin or control and found no reduction in the incidence of DGE. In a separate study, erythromycin did significantly reduce postoperative DGE, but the study was very small, and DGE occurred in 57% of patients in the control arm (Ohwada et al, 2001). Tani and colleagues (2006) randomized patients to retrocolic or antecolic gastrojejunostomy and found a significantly lower rate of DGE with the antecolic approach.
Although DGE and pylorus preservation have been a concern, to date no study has found a difference in the incidence of DGE following conventional compared with pylorus-preserving procedures (Diener et al, 2007). As discussed earlier in this chapter, and as highlighted in Table 25.7, the reported incidence of DGE was lower in two studies analyzing anastomotic technique. Bassi and colleagues (2005b) found a statistically significant reduction in DGE in patients who had PG compared to PJ. In the study by Yeo and others (2002), comparing radical with standard PD, an increase in DGE was seen in the radical group. It is important to note, however, that neither study had DGE as the primary end point.
Among these studies, significant variability was observed in the range of reported incidence of DGE. As with pancreatic fistula, part of the variability in incidence across studies was from the variation in definition. The ISGPF recently devised a formal grading system for DGE based on duration of nasogastric tube placement and the timing of patients’ tolerance of solid food (Wente et al, 2007).
Hemorrhage
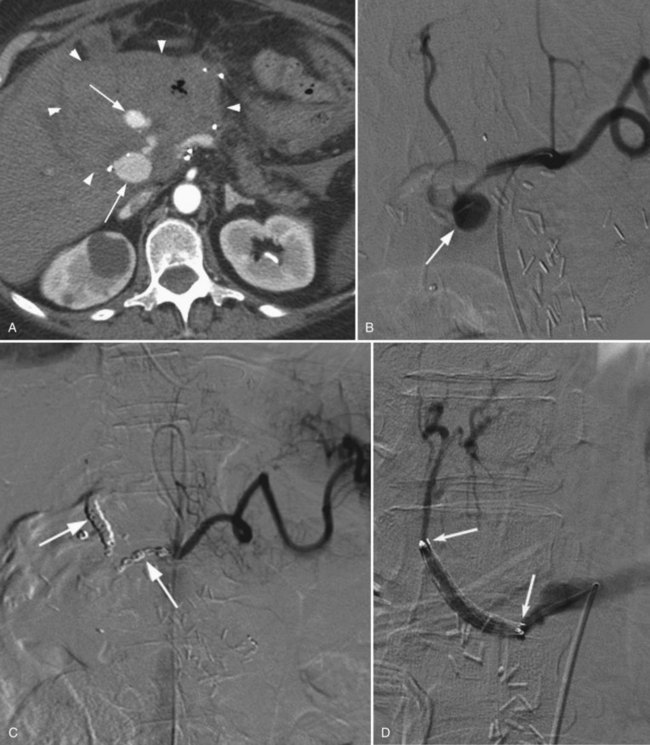
Postoperative hemorrhage is a potentially lethal complication after pancreatectomy, and it can occur in the immediate postoperative period (<7 days) or in a delayed fashion (>14 days). Management is often guided first by the patient’s hemodynamic stability. Any unstable patient should be transfused and taken for reexploration. Patients who can be easily stabilized may be candidates for endoscopic or interventional radiologic management. Intraluminal bleeding is most commonly from the gastrojejunal anastomosis and can often be managed with endoscopic therapy. Extraluminal bleeding from arterial pseudoaneurysm (Fig. 25.3) or vascular erosion from a pancreatic fistula typically occur later, following operation, and these can be approached via angiographic embolization in stable patients. A high index of suspicion is critical, as mortality from late postoperative hemorrhage is significant (Brodsky & Turnbull, 1991).
Yekebas and others (2007) analyzed the treatment and outcome of 87 patients who had postoperative hemorrhage in a series of 1669 pancreatic resections in 1524 patients (overall hemorrhage rate, 6%). Hemorrhage was classified as mild in 36 patients (41%) and severe in 51 patients (59%); 29 patients (33%) had a sentinel bleed. The bleeding site was extraluminal in 51 (59%) and intraluminal in 36 (41%); 17 patients had what appeared to be extraluminal hemorrhage, but further evaluation confirmed bleeding from the transected pancreas or resection cavity; and 34 patients (39%) had pancreatic fistula that preceded hemorrhage.
This and other studies have demonstrated that significant morbidity and mortality are associated with postoperative hemorrhage, especially when delayed beyond postoperative day 5 (Choi et al, 2004). These patients often require reoperation or interventional radiology procedures to halt life-threatening hemorrhage. Furthermore, patients who experience a postoperative hemorrhage often have underlying complications that require treatment, such as pancreatic fistula. If angiographic embolization successfully treats hemorrhage, cross-sectional imaging should be performed to assess for the presence of leak, fistula, or abscess that would require drainage.
Imaging and Interventional Therapy of Complications after Pancreatic Surgery
Imaging of Complications After Pancreatic Surgery
Imaging plays an important role in identifying complications and directing therapy in patients who have recently undergone pancreatic resection. The observation that 22% of patients undergoing a PD over a 12-year period required percutaneous interventions highlights the importance of imaging and image-guided interventions in the postoperative management of these patients (Zink et al, 2009). Transabdominal ultrasound (US) is a commonly available imaging tool that enables detection of focal, encapsulated, or free intraperitoneal fluid collections, but gastrointestinal gas and/or postoperative pneumoperitoneum superimposition represents the major disadvantage of US in the evaluation of the pancreas (see Chapter 13).
Computed tomography (CT) is the most effective imaging modality for evaluation of the pancreas after surgery (Johnson et al, 2002; see Chapter 16). It allows detection of the normal features as well as postoperative complications such as bleeding, anastomotic leakage, abscess, or fistula formation (Johnson et al, 2002; Scialpi et al, 2005
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree