Table 36.2 Randomized controlled trials of β-blockers versus non-active treatment for the prevention of first variceal bleeding.
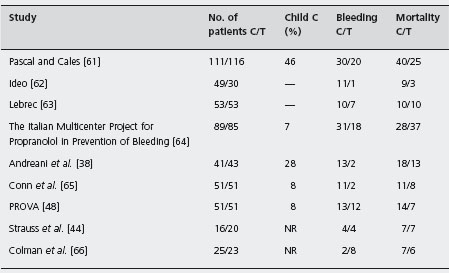
C: control; T: β-blockers; NR: not reported.
β-Blockers versus nitrates and beta blockers
A randomized trial first published in 1996 [84] and further evaluated in 2000 [85] showed that nadolol and isosorbide dinitrate combined were more effective than nadolol alone for prevention of first variceal bleeding.
In this study [85] 146 patients with cirrhosis and known esophageal varices, but no bleeding, were treated for seven years. Sixteen in the nadolol group and eight in the combination group bled (logrank test, p = 0.02). The cumulative bleeding risk was 29% and 12% respectively (95% CI for the difference 1–23%). Addition of isosorbide 5-mononi-trate did not increase the incidence of liver failure, development of ascites or renal insufficiency; five patients requested discontinuation of nitrates due to adverse effects. However, the results of the most recent multicenter and larger randomized controlled trial are conflicting. In this study 349 cirrhotic patients with gastroesophageal varices were randomized to receive propranolol + placebo (n = 174) or propranolol + isosorbide mononitrate (ISMN) (n = 175) [86]. There were no significant differences in the one and two-year actuarial probability of variceal bleeding between the two groups (propranolol + placebo 8.3% and 10.6% respectively; propranolol + ISMN, 5% and 12.5% respectively). Survival was also similar. Adverse effects were significantly more frequent in the propranolol + ISMN group, mainly due to a greater incidence of headache. The combination was otherwise safe and did not produce any deleterious effects on renal function.
β-blockers effectively prevent variceal bleeding. Adding nitrates does not further decrease the low residual risk of bleeding in patients receiving propranolol. Current data do not support a recommendation for additive medication to non-selective β-blockers for primary prophylaxis of variceal bleeding. Ald
Variceal ligation versus β-blockers
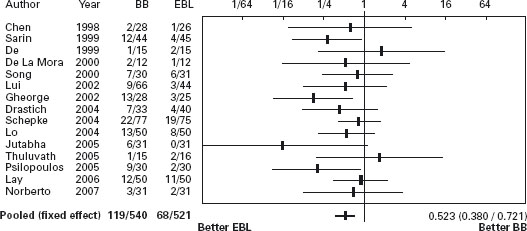
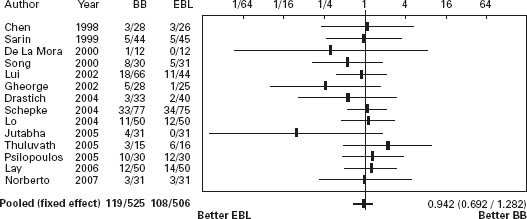
There are 15 randomized trials comparing endoscopic band ligation of high risk esophageal varices to propranolol [58, 70–83] (see Table 36.3). In the first trial, Sarin et al. found that ligation was more effective than propranolol for prevention of bleeding (actuarial survival, propranolol 43%, ligation 15%, p < 0.05) [71]. However, the rate of bleeding in the propranolol group was higher than has been observed in some other studies. This may be because of the lower mean dose of propranolol used (70mg/day compared with 123mg/day in previous studies). Alternatively, the difference in bleeding rates between groups may have occurred because of the relatively small number of patients studied and the resultant rather wide confidence intervals. The rate of bleeding in the propranolol group was the same as that in the non-treated group in a previous trial by the same authors in which the same selection criteria were used [53]. In the meta-analysis of the 15 studies variceal ligation significantly reduced the risk of first variceal bleeding compared with propranolol (POR 0.52; 95% CI: 0.38–0.72). However, the same meta-analysis did not find any difference in terms of mortality, with a trend in a favor of drug treatment (POR 0.94; 95% CI: 0.69–1.28), and iatrogenic bleeding due to ligation causing death [57,59]. Recently Wang et al. reported similar efficacy of ligation to the combination of nadolol plus ISMN (see Figures 36.1 and 36.2) [87]. Ala
Table 36.3 Randomized controlled trials of variceal ligation versus β-blockers for the primary prophylaxis of variceal bleeding.
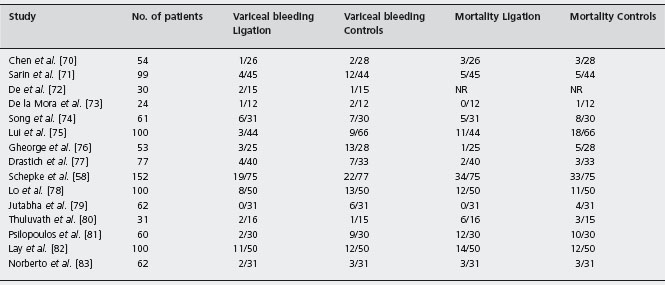
NR: not reported.
Figure 36.1 Primary prevention: Forrest plot of randomized trials of banding ligation of esophageal varices versus beta-blockers in primary prevention of variceal bleeding. Portal hypertensive bleeding. BB: beta-blockers; EBL: Endoscopic band ligation. Data are expressed as OR (95% CI) in a log scale.
The conflicting results of these studies and the small number of patients randomized and events observed, as well as the cost of endoscopic variceal ligation, do not provide sufficient evidence for recommending any change in the current practice of prescribing propranolol as the treatment of first choice for the primary prevention of variceal bleeding and using band ligation if there are contraindications or intolerance to beta-blockers. Ala
Variceal ligation versus sclerotherapy
Variceal ligation was compared with sclerotherapy for the primary prevention of variceal bleeding in three small studies [52, 60, 88] of which one was published only in abstract form [52]. The results were conflicting. One study indicated that sclerotherapy was more effective [88], the second that ligation was more effective [60], and the third that the two interventions are of similar efficacy [52]. Thus, it is not surprising that there is significant heterogeneity in the meta-analysis (p = 0.045) for bleeding, and combining the data in a meta-analysis may not be justified. There was no significant difference for mortality (POR 0.84; 95% CI: 0.35–2.05).
Figure 36.2 Primary prevention: Forrest plot of randomized trials of banding ligation of esophageal varices versus beta-blockers in primary prevention of variceal bleeding. Mortality. BB: beta-blockers; EBL: Endoscopic band ligation. Data are expressed as OR (95% CI) in a log scale.
Conclusion
The data from prophylactic trials suggest that screening for varices in cirrhotic patients should be part of routine clinical practice, and if these are found, prophylactic treatment to prevent first variceal bleeding should be offered [89, 90]. Shunt surgery prevents bleeding but the increase in mortality and the long-term risk of encephalopathy make this treatment unacceptable. Prophylactic sclerotherapy should not be used as it is relatively ineffective, costly and potentially dangerous. The treatment of first choice is prophylactic β-blocker therapy; it is inexpensive, easy to administer, and effective for prevention of the first variceal hemorrhage and of bleeding from gastric mucosa. Primary prophylaxis with variceal ligation appears to be safe and may be a reasonable alternative for: (1) patients with contraindications to β-blockers; (2) patients who cannot tolerate or have no hemodynamic response to the drug therapy. However, it is unlikely to be a routine prophylactic treatment as it is much more expensive and less available than β-blockers and it will not prevent gastric mucosal bleeding [57]. The trend towards increased mortality with banding needs further observation, as increased mortality was seen with prophylactic sclerotherapy.
Outcome of acute variceal bleeding
Acute variceal bleeding is a life-threatening complication in patients with cirrhosis and portal hypertension, with mortality that ranges from 10% to 40% [91]. Although overall survival is improving [1], because of new therapeutic approaches, mortality is still closely related to failure to control hemorrhage or early re-bleeding, which is a distinct characteristic of portal hypertensive bleeding and occurs in between 15% and 50% of patients in the first days to six weeks after admission [92].
HVPG has been shown to be prognostic for both survival and the course of acute bleeding. In 1986 Vinel et al. documented that short term prognosis in alcoholic cirrhotics with variceal bleeding was independently associated with portohepatic gradient measured within 48 hr of admission [93]. This was confirmed in a small study of 22 patients, in which the best cutoff for continued bleeding or early re-bleeding was HVPG > 16mmHg [94]. Villanueva et al. showed that HVPG > 20mmHg and a decrease ≤10mmHg under vaso-active therapy were independent predictors of further bleeding [95]. An HVPG > 20mmHg has been shown to correlate with important clinical outcomes, such as more difficulty in controlling acute variceal bleeding, more early re-bleeding, more blood transfusion need, more days in intensive care and increased hospital mortality [96]. Avgerinos et al. showed that HVPG > 16mmHg was independently associated with death and/or early re-bleeding, evaluating HVPG measurements before and immediately after endoscopic treatment and every 24 hours for a five-day period [97]. Interestingly, there was sustained rise of portal pressure after sclerotherapy, but not band ligation. In addition, Vlachogiannakos et al. showed that somatosta-tin but not octreotide effectively prevents the post endoscopic increase of HVPG [98]. With the prognostic association of high HVPG with the course of bleeding (using vasoactive drugs and endoscopic therapy) and mortality, a randomized study showed that urgent TIPS in patients with HVPG > 20mmHg, protected against continued or repeated bleeding and reduced mortality [96]. This study clearly demonstrated the therapeutic benefit of intervention based on HVPG measurement and validated the value of 20mmHg HVPG as a cutoff. The question then arises about the applicability of measuring HVPG in this setting, which although accurate and reproducible, with little co-efficient of variation in measurement, and with few complications is not currently feasible outside a research setting [99].
Several studies have evaluated factors associated with failure to control bleeding and mortality. A consensus has determined that control of variceal bleeding spans the first five days and mortality is death within six weeks of onset of bleeding [89]. Ben-Ari et al. showed that in 385 cirrhotics treated initially with vasoactive drugs and then endosopic therapy if bleeding continued or recurred (within five days) active bleeding at endoscopy (independent of the interval from onset of melena and/or haematemesis or the interval to admission to hospital), the severity of liver disease (mainly Child-Pugh grade C) as well as encepha-lopathy, platelet count and history of alcoholism were all independently associated with failure to control bleeding (internal validation of the model was performed) [100]. There were some interactions between variables in that active bleeding was associated with transfusion need and transfusion need was associated with Child-Pugh class, and shorter interval to admission. Interestingly a higher Child-Pugh score and increased mortality was independent of transfusion requirement. Independent factors associated with mortality within 30 days were failure to control bleeding within five days, raised bilirubin, encephalopa-thy, shorter interval to admission to hospital and plasma urea. Lecleire et al. evaluated prospectively 468 consecutive patients with cirrhosis and upper gastrointestinal bleeding (23.5% died during hospitalization) [101]. The independent factors associated with in hospital mortality were: presentation with hematemesis, bleeding starting in hospital, pro-thrombin time <40% (the strongest association, reflecting severity of liver disease), recent use of steroid drugs within seven days of bleeding, age >60 years and a concomitant hepatocellular cancer. Thomopoulos et al. retrospectively evaluated 141 patients with acute variceal bleeding (18.6% 6 week mortality) treated with somatostatin started before endoscopy and endoscopic ligation [102]. Early re-bleeding, Child-Pugh grade C and shock at admission were independent predictors of mortality at six weeks, whilst active bleeding and presence of infection (prophylactic antibiotics were not routinely used) were not adverse factors.
Lo et al. also found active bleeding to be independently associated with recurrent bleeding, but not mortality, although this was higher in the group with active bleeding at endoscopy [103]. In addition, the presence of hepatocellular carcinoma may alter the immediate prognosis [101, 104, 105] so that early imaging (large tumors will be detected by a bedside ultrasound) to detect HCC may alter the therapeutic algorithm, for example TIPS rescue therapy may not be indicated and only vasoactive and endoscopic methods applied. A concomitant portal vein thrombosis, whether due to tumor or not may also worsen prognosis as often bleeding is more difficult to control.
The use of prophylactic antibiotics should be standard therapy for patients with cirrhosis who have variceal bleeding [106]. The association with failure to control was shown prospectively [107], following a hypothesis that proposed infection as a trigger for bleeding [108], and the causal association was shown in two randomized trials [109,110]. Antibiotics result in better control of bleeding and less early re-bleeding. Alc The prognostic models that assess control of bleeding should now be based in cohorts of patients who have had prophylactic antibiotics.
In a prospectively evaluated cohort of 117 patients with cirrhosis [111], HVPG was measured within 48 hours. As in a previous study HVPG > 20mmHg was independently predictive of failure to control bleeding at five days, together with shock (systolic blood pressure at admission <100mmHg) and non-alcoholic etiology of cirrhosis, giving an optimal discrimination using an AUROC curve with a c statistic of 0.79 [96]. However, removing HVPG, the model contained Child-Pugh class, shock at admission and non-alcoholic cirrhosis as independent associations with a c statistic of 0.8, that is, the same discriminatory capacity. The authors devised a simple point score which will be useful in clinical practice as it identifies a subgroup with 40% or more chance of failure to control variceal bleeding. Indeed the authors confirm what is already known clinically about these patients with difficult bleeding with the added data; this is associated with a higher HVPG [89]. This should intensify clinical research into more effective vasoactive regimens to lower portal pressure and treatment algorithms which will result in more effective therapy to control bleeding ab initio, for example early TIPS after diagnostic endoscopy, or first-line injection with glues at diagnostic endoscopy, or indeed the recently described self-expanding covered esophageal stent [112]. A randomized trial of early TIPS, reproposing a management scheme used by Orloff et al. with emergency porto-caval shunt [113].
Although survival following variceal bleeding has progressively improved [91,114], it remains to be proven that “complete” or better control of bleeding with early TIPS or other measures over and above what is current practice after failed vasoactive drugs and endoscopy will result in improved survival. In the large study by Ben-Ari et al. mortality in Child-Pugh grade C was independent of transfusion need, that is, of severity of bleeding [100]. Thus there may be an inexorable train of events which is initiated by bleeding in this group of patients, leading to death, independent of good control of bleeding. This concept of “going past the point of no return” is seen in the development of renal failure, despite the prompt resolution of sepsis (not associated with spontaneous bacterial peritonitis) in cirrhosis, where just over 20% of such patients have the complication of renal failure despite optimal treatment and resolution of the precipitating septic event [115].
Intravenous third-generation cephalosporines, compared to oral quinolones are a good option for prophylaxis in upper GI bleeding in cirrhosis being active against Gram-negative bacteria and non-enterococcal streptococci [116]. In addition, many cirrhotics receive quinolones already as prophylaxis for spontaneous bacterial peritonitis, and quinolone resistant bacteria are currently sensitive to cephalosporins. Current trials have started antibiotics after endoscopic diagnosis. Possibly the administration should be started at admission before endoscopy with the potential to increase therapeutic benefit.
The clinician should be aware that most clinical trials have focused on esophageal varices, with very few designed to evaluate therapy for gastric varices. Gastric varices may lead to more severe bleeding initially, and tend to re-bleed frequently [117]. The following sections refer to esophageal varices unless specified.
Randomized controlled trials for the treatment of acute variceal bleeding
Pharmacologic treatment
Vasoactive drug treatment is the only treatment that does not require sophisticated equipment or the skills of a specialist and is immediately available, even before the patient is admitted to hospital [118]. Furthermore, as evidence suggests that those patients with high variceal or portal pressure are likely to continue to bleed or re-bleed early, prolonged drug therapy that lowers portal pressure over days may be the optimal treatment [8,119]. The vasoactive drugs that are currently used in the management of acute variceal bleeding are vasopressin, glypressin, somatostatin, octreotide and vapreotide. Vasopressin, which is a powerful vasoconstrictor, lowers portal pressure through the induction of smooth muscle contraction, particularly in splanchnic arterioles. However, the drug also causes systemic vasoconstriction which leads to serious side effects, such as cardiac arrhythmias, myocardial ischemia, mesenteric ischemia and cerebrovascular episodes, resulting in cessation of therapy in up to 25% of cases [120,121].
Terlipressin is a synthetic analogue of vasopressin (trigly-cyl lysine vasopressin). It has an intrinsic effect as well as being converted in vivo into vasopressin by enzymatic cleavage of the triglycyl residues. This prolongs its biological half-life, so that a continuous intravenous infusion is unnecessary. Somatostatin has been used in the pharmacological treatment of variceal bleeding because it reduces splanchnic blood flow [122], portal pressure and azygous blood flow in patients with cirrhosis [123], although only the findings regarding the reduction in azygous flow are consistent. Bolus injections of somatostatin appear to have greater hemodynamic effects as compared with continuous infusion. Finally, octreotide has been reported to cause a reduction in portal pressure [124], and a transient decrease in azygous blood flow [125], but there are some studies that do not confirm these data, using similar or even greater doses of the drug [126].
Randomized controlled trials of vasoactive drug treatment of acute variceal bleeding
Drugs versus placebo
See Table 36.4.
Vasopressin versus placebo
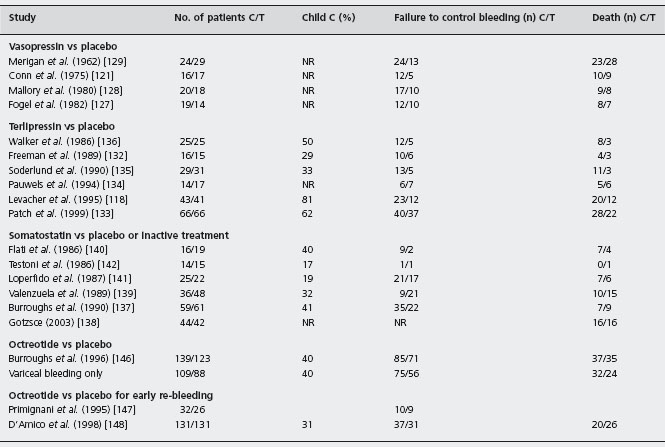
Vasopressin was compared with non-active treatment or placebo in four randomized controlled trials [121,127–129], including only 157 patients. In two trials the intra-arterial route of administration was used [121, 129]. There was a significant heterogeneity in the evaluation of failure to control bleeding. There was a clear trend in favor of vasopressin but the result was not statistically significant by the Der Simonian and Laird method (POR 0.23; 95% CI: 0.05–1.02). Moreover there was no difference in mortality (POR 0.98; 95% CI: 0.47–2.1). Complications were reported in up to 64% of patients, which led to discontinuation of treatment in 25% of cases. In order to minimize the systemic complications of vasopressin, nitroglycerin has been added to the regimen. This drug is a powerful venous dilator, reduces the portal vascular resistance and improves myocardial performance. Three randomized controlled trials have compared vasopressin alone with vasopressin plus nitroglycerin (transdermally [120], sublingually [130], and intravenously [131]), including 176 patients. Failure to control bleeding was significantly less common with vasopressin plus nitroglycerin (POR 0.39; 95% CI: 0.21–0.72) but there was no difference in mortality (POR 0.94; 95% CI: 0.49–1.79). In two of the trials [130, 131], adverse effects were significantly reduced with the combination treatment. However, nitroglycerin, because of portocollateral shunting, bypasses the liver, and can cause significant systemic effects. Hence, this combination therapy must be monitored very closely and is less applicable as an immediate therapy.
Table 36.4 Randomized controlled trials of drugs versus placebo for the treatment of the acute bleeding episode.
C: placebo; T: drug; NR: not reported.
Terlipressin versus placebo
The clinical efficacy of terlipressin has been evaluated in six randomized placebo-controlled studies [118, 132–136] including 388 patients. In two studies endoscopic sclero-therapy was employed at the initial diagnostic endoscopy [118,133]. In one trial the drug was given while the patient was transferred to hospital. There was a statistically significant reduction in failure to control bleeding with terlipressin compared with placebo (POR 0.49; 95% CI: 0.33–0.75) and more importantly the same meta-analysis showed that terlipressin is the only vasoconstrictor that significantly reduces mortality (POR 0.51; 95% CI: 0.33–0.79). However, there is some criticism of these studies. The sample sizes were small, allowing a large type 2 error in the first three trials [132, 135,136], and the evidence in the early administration trial [118] of the effect of terlipressin, given only as three doses up to eight hours, does not readily explain the apparent benefit on mortality (only in group C patients) via an effect on the control of bleeding. Alc
Somatostatin versus placebo
Three placebo-controlled trials of somatostatin exhibit divergent results [137–139]. The trials by Valenzuela et al. [139] and Gotzse et al. [138] suggested that somatostatin was no more effective than placebo. Unfortunately both studies had a very long recruitment period, suggesting marked patient selection. Moreover, Gotzse et al. did not evaluate the endpoint of failure to control bleeding [138], while Valenzuela et al. reported an extremely high response rate (83%) in the placebo group (the highest ever reported) [139]. In contrast, the study by Burroughs et al. reported a statistically significant benefit for somatostatin in controlling variceal bleeding over a five-day treatment period [137]. These differences in the reported results caused statistically significant heterogeneity (p = 0.006) in the meta-analysis of the six studies which compare somatostatin with placebo or inactive treatment [140–142].There was a trend in favor of somatostatin but the result was not statistically significant by the Der Simonian and Laird method (POR 0.6; 95% CI: 0.21–1.65). There was no difference in mortality between the two treatment groups (POR 1.02; 95% CI: 0.64–1.61). Alc
Moitinho et al. evaluated a total of 174 patients with acute variceal bleeding who were randomized to receive somatostatin for 48 h: (A) one 250 μg bolus +250μg/h infusion; (B) three 250 μg boluses +250μg/h infusion; (C) three 250μg boluses +500μg/h infusion [143]. The 500μg/h infusion dose achieved a higher rate of control of bleeding (82 vs 60%, p < 0.05), less transfusions (3.7 +/– 2.7 vs 2.5 +/– 2.3 units, p = 0.07) and better survival (93 vs 70%, p < 0.05) than schedules A and B. Others have confirmed the above results using somatostatin 500μg/h [144,145]. Ald
Octreotide versus placebo
There is only one double blind randomized trial of octreotide versus placebo (n = 262) in the management of acute variceal bleeding, currently available only in abstract form [146]. In this study a continuous five-day infusion of 50μg/ hour octreotide, started as soon as possible after admission was not more effective than placebo, whether or not injection sclerotherapy was needed for active bleeding or drug failure. Infections were treated if suspected on clinical grounds and were similar in the two trial groups. Moreover, two other studies using octreotide (100 μg eight-hourly, subcutaneously) or placebo after the control of the initial bleeding episode failed to show any difference in early re-bleeding or mortality between the two treatment groups [147,148].
Drugs versus balloon tamponade
Six trials compared vasoactive drugs with balloon tamponade. The drugs were terlipressin in three studies [149–151], somatostatin in two studies [152,153] and octreotide in one study [154]. Meta-analysis of these six trials showed that the drugs were as effective as balloon tamponade for prevention of failure to control bleeding (POR 1.04; 95% CI: 0.63–1.72) or death (POR 0.65; 95% CI: 0.36–1.16). Alc Sensitivity analysis showed that there was no difference according to the type of drug. However, the sample sizes were small and the endpoints not very clear, indicating that these results should be interpreted with caution. Tamponade, if used properly, provides good control of bleeding. However, the balloons should not be inflated for more than 12 hours and preferably less, and bleeding frequently recurs when the balloons are deflated. From the trial reports it is not always clear when efficacy is being assessed, for example during therapy or at the end of an interval of 24 hours after termination of drug therapy or tamponade.Whether the new expanding oesophageal stent will replace tamponade will be known from RCTs [112].
Drugs versus drugs
See Table 36.5.
Terlipressin versus vasopressin
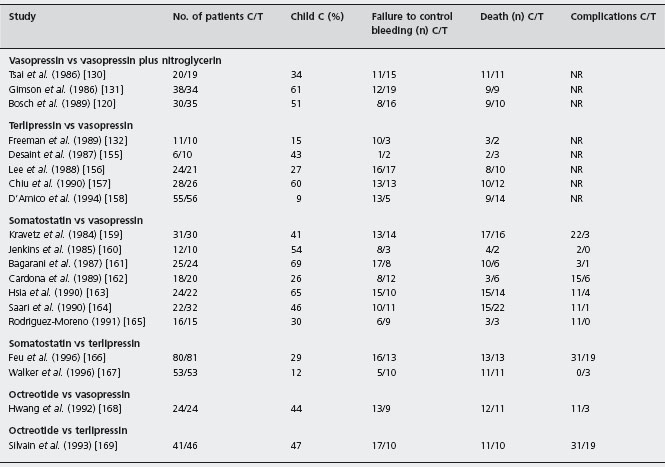
Terlipressin was compared with vasopressin in five small, unblinded studies involving only 247 patients [155–158, 170]. In two studies, vasopressin was associated with nitro-glycerin [157, 158]. Failure to control bleeding was less frequent with terlipressin, but the result was not statistically significant (POR 0.74; 95% CI: 0.36–1.14). There was no difference in mortality between the two treatment arms (POR 1.48; 95% CI: 0.85–2.57). More importantly, the complication rate was significantly lower with terlipressin even when vasopressin was combined with nitroglycerin. Alc
Somatostatin versus vasopressin
Somatostatin was compared with vasopressin in eight trials including 343 patients [159–165,171]. Although these trials showed a trend in favor of somatostatin, the difference was not statistically significant (POR 0.74; 95% CI: 0.48–1.13). There was no difference in mortality between the two vasoactive agents (POR 0.97; 95% CI: 0.6–1.5). However, a statistically significant reduction in complications was observed in the group receiving somatostatin (POR 0.11; 95% CI: 0.07–0.19) as the mean complication rate was 51% with vasopressin and only 10% with somatostatin. Alc
Somatostatin versus terlipressin
Three studies have compared somatostatin with terlipressin involving 326 patients [166, 167, 172]. These studies showed that the two drugs were similarly effective in preventing failure to control variceal bleeding and death. Moreover, in one of these studies a significantly lower incidence of complications in the somatostatin group was reported [166]. [Alc]
Octreotide versus other drugs
The efficacy of octreotide treatment in comparison to other vasoactive drugs, for acute variceal bleeding, has not been adequately evaluated. Octreotide was found to be comparable to vasopressin in two low quality studies (n = 89 in total) [168, 173], and to terlipressin plus nitroglycerin in another (n = 87 patients) [169]. However, the sample sizes were small and the trials may have lacked power to show differences, and the endpoints are not very clear, indicating that these results should be interpreted with caution.
Somatostatin versus other drugs
In a single center randomized trial 62 cirrhotics with variceal bleeding were randomized to receive somatostatin (n = 35) versus somatostatin plus nitroglycerine (n = 26) [174]. Similar proportions of patients achieved control of bleeding in the two groups (88.6% vs 92.3% respectively). The authors concluded that the addition of nitrates does not improve the control of bleeding or prevent early re-bleeding. Ald
Table 36.5 Randomized controlled trials of comparisons between drugs for the treatment of the acute bleeding episode.
Recombinant activated factor VIIa
In a placebo-controlled double blind randomized trial, recombinant factor VIIa was safe but no clear-cut benefit in control of bleeding or mortality was seen [175]. More recently a randomized controlled trial assessed the efficacy and safety of rFVIIa in patients with advanced cirrhosis and active variceal bleeding [176]. At 31 hospitals in an emergency setting, 256 patients (Child-Pugh > 8; Child-Pugh B = 26%, C = 74%) were randomized equally to: placebo; 600μg/kg rFVIIa (200 + 4x 100μg/kg); or 300 μg/ kg rFVIIa (200 + 100μg/kg). The primary composite end-point consisted of failure to control bleeding within 24 hours, or failure to prevent re-bleeding or death by day five. Administration of rFVIIa had no significant effect on the composite endpoint compared with placebo (p = 0.37). There was no significant difference in five-day mortality between groups; however, 42-day mortality was significantly lower, with 600 μg/kg rFVIIa compared with placebo (odds ratio 0.31; 95% CI: 0.13–0.74), and bleeding-related deaths were reduced from 12% (placebo) to 2% (600 μg/ kg), but this was not associated with differences in control of bleeding. There is no evidence that recombinant activated factor VIIa improves outcome in acute variceal bleeding. Alb
Randomized controlled trials of emergency sclerotherapy in the management of acute variceal bleeding
Injection sclerotherapy, first introduced in 1939 and “rediscovered” in the late 1970s, has been used for the control of acute variceal bleeding over the past two decades. Paradoxically the best evidence for the value of sclerotherapy in the management of acute variceal bleeding has come from a more recently published study by the Veterans Affairs Cooperative Variceal Sclerotherapy Group [177]. In this study sclerotherapy, compared with sham sclerotherapy, stopped hemorrhage from actively bleeding esopha-geal varices (91% in sclerotherapy arm compared with 60% in sham sclerotherapy, p < 0.001, ARR = 29%, NNT = 3) and significantly increased hospital survival (75% vs 51%, p = 0.04 ARR = 24%, NNT = 3). Ald
Today it is generally accepted that sclerotherapy should be carried out at the diagnostic endoscopy, which should take place as soon as possible, because there is evidence that this is beneficial compared with delayed injection [178, 179]. No more than two injection sessions should be used to arrest variceal bleeding within a five-day period [92]. Several sclerosing agents have been used for injection, polidocanol 1–3%, ethanolamine oleate 5%, sodium tetra-decyl sulfate 1–2% and sodium morrhuate 5%. There is no evidence that any one sclerosant can be considered the optimal sclerosant for acute injection, as it has been shown that a substantial proportion of intravariceal sclerosant ends up in the paravariceal tissue and vice versa. B4 There is no evidence that one technique is better than the other. One of the main shortcomings of sclerotherapy is the risk of local and systemic complications, although this varies greatly between trials and may be related to the experience of the operator [180].
Sclerotherapy plus drugs/balloon tamponade versus drugs/balloon tamponade
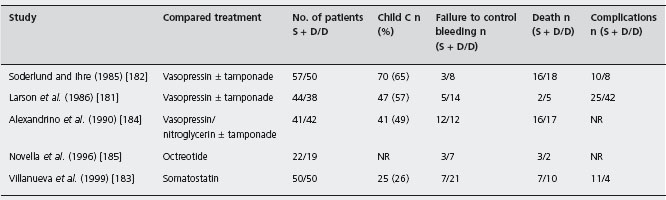
The five trials, three papers [181–183] and two abstracts [184,185] comprised 400 patients (413 episodes): three used vasopressin [181,182,184], one somatostatin [183] and one octreotide [185]. Treatment effect was evaluated within 24 hours up to 120 hours, except in one study at two weeks [181]. Specific definitions of failure to control bleeding are given in four [181–183, 185]; only three [181, 182, 186] report complications; blood transfusion was reported in four [181–183,185]; three report less transfusion with sclerotherapy [181–183]; and one no difference [185]. Median quality score was 58 (49–68.5). Failure to control bleeding was reported: 214 episodes of variceal bleeding in the sclerotherapy and vasoactive drugs/balloon tamponade group and 199 in the vasoactive drugs/balloon tamponade alone. Failure to control bleeding was significantly more frequent without sclerotherapy, with pooled difference being 16.3% (95% CI: 8.7–23.9%; p = 0.0001)). Alc The NNT was 6 (95% CI: 4–11). The publication bias assessment was 15 null or negative studies. The sensitivity analyses differed from the main metanalysis as follows: abstracts alone showed no differences between trial groups. In only one study, octreotide added to sclerotherapy did not improve control of bleeding. Fewer deaths occurred with sclerotherapy combined with drugs than with drugs/balloon tamponade alone (Q, p = 1.0), with a 5.5% difference (95% CI: –1.8–12.7%; p = 0.138) with no significant changes in the sensitivity analyses. Complications: reported in three studies [181–183] and two [181, 183] did not report per patient. A systematic evaluation was not possible (see Table 36.6).
Sclerotherapy versus drugs
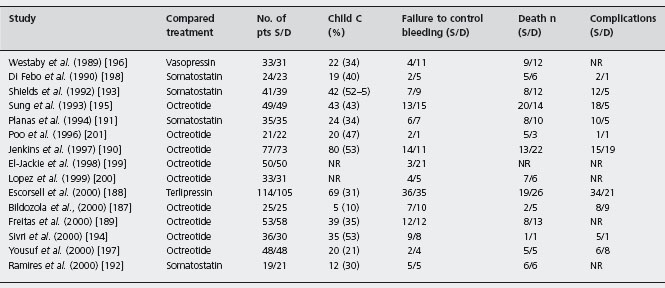
There are 15 studies comprising 1296 patients (1324 episodes), 11 papers [187–197] and four abstracts [198–201]; one used vasopressin [196], one used terlipressin [188], four used somatostatin [191–193,198] and nine used octreotide [187,189,190,194,195,197,199–201]. Treatment effect was evaluated at the end of drug infusion (12–168 hours). The efficacy of sclerotherapy was 83% (57–94%) in studies of 12–48-hour drug infusion [189–192, 194–196, 198–201] and 73% (68–83%) when 120–168 hours [187,188,193]. All except three trials [189,199, 200] report complications. One study had less transfusion with sclerotherapy (units, 2.1 ± 0.41 vs 2.9 ± 0.79; p < 0.05) [199], ten found no difference [187–195,197], one study did not state [201], and three had no report [196,198, 200]. Median quality score was 60 [51–73]. Considering failure to control bleeding, there was significant heterogeneity (p = 0.01), but only in the magnitude and not in the direction of treatment effect; in only two were drugs better than sclerotherapy, but without reaching statistical significance [190, 201]. Excluding abstracts there was no heterogeneity (p = 0.4). Failure to control bleeding was less frequent with sclerotherapy with a difference of 3.4% (95% CI: –1.8–8.5%; p = 0.2) with no change following sensitivity analyses. If all papers are considered then sclerotherapy was more effective, with a 5.9% difference (95% CI: 1.5–10.3%) (p = 0.008). The other sensitivity analyses showed that octreotide in nine studies (p = 0.04) and vasopressin (one study) as well as the combined group of somatostatin and octreotide were significantly worse than sclerotherapy. In the trials there were no differences between treatment groups considering only patients with cirrhosis, or abstracts, or somatostatin, or drug infusion more or less than 120 hours, or combined group of vasopressin and terlipressin or terlipressin alone. There were fewer deaths with sclerotherapy – 4.3% (95% CI: 0.6–8.1%) (p = 0.02). Ala The NNT was 23 (95% CI: 12–157). The effect is not robust, as publication bias assessment was four null or negative studies. Sensitivity analyses did not change the main meta-analysis. Complications in 12 studies [187,188,190–198,201] had significant heterogeneity (p = 0.0001) and were less frequent with any drug 8.8% (95% CI: 0.2–15.6%; p = 0.01) (see Table 36.7).
Table 36.6 Randomized controlled trials of sclerotherapy plus drugs/balloon tamponade versus drugs/balloon tamponade alone for the treatment of the acute bleeding episode.
S + D/D: sclerotherapy + drugs/drugs; NR: not reported.
Table 36.7 Randomized controlled trials of sclerotherapy versus drugs for the treatment of the acute bleeding episode.
S: sclerotherapy; D: drugs; NR: not reported.
Sclerotherapy plus drugs versus sclerotherapy alone
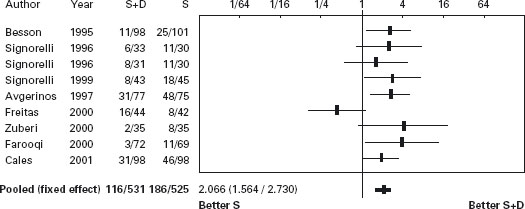
The eight trials, six papers [189, 202, 205–208] and two abstracts [203,204] (the first with two treatment arms) comprised 1026 patients; four studies were placebo controlled [202, 203, 205, 206, 208]. Vasoactive drug was administered for 120 hours in six trials [202–206,208] and 48 hours in two [189,207]. The efficacy of sclerotherapy was 82.5% (81–84%) with 48 hour drug administration and 61.5% (36–77%) with 120 hours (p = ns). In all studies but one the average units transfused were significantly less with combination therapy [189]. Median quality score was 60 (49.5–75). Failure to control bleeding was less frequent with sclerotherapy combined with drugs: 13.2% (95% CI: 8.4–18.1%; p < 0.0001). Ala The NNT was 8 (95% CI: 5–15). The publication bias assessment was 47 null or negative studies, that is, the result is very robust. In the sensitivity analyses, terlipressin did not have an advantage: there was no advantage of any drug added to sclerotherapy for 48 hours administration and no advantage of combined therapy when no placebo drug was used. The difference in mortality was 3.4% (95% CI: –0.4–7.1%) in favor of combined therapy (p = 0.08). Sensitivity analysis showed that in placebo controlled trials in which drug was given for 120 hours the difference was 1.3% (95% CI: –3.3–5.8%), strongly suggesting no benefit in mortality despite benefit for control of bleeding. For complications, five studies had data but only two reported per patient [202, 205], with no statistical differences (see Table 36.8 and Figures 36.3 and 36.4).
Table 36.8 Randomized controlled trials of sclerotherapy plus drugs versus sclerotherapy alone for the treatment of the acute bleeding episode.
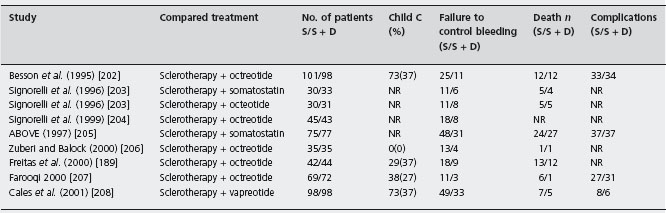
S: sclerotherapy; S + D: sclerotherapy plus drugs; NR: not reported.
Figure 36.3 Acute bleeding: Forrest plot of risk difference for control of bleeding in trials of sclerotherapy (S) vs sclerotherapy combined with vasoactive agents (S + D).
Sclerotherapy versus variceal ligation
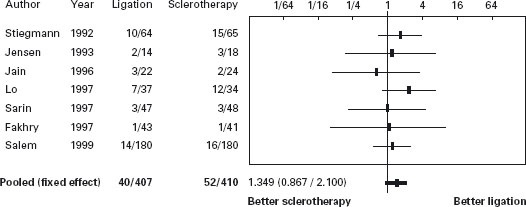
The twelve trials, eight papers [209–216] and four abstracts [217–220], comprised 1309 patients. In seven trials only patients with cirrhosis were included [209–213, 216, 219]. The same ligating device (Bard Interventional Products, Tewksbury, Massachusetts) with an overtube was used in eight [209–212, 214–216]; in three this was not mentioned [217–219] and one used a six-shooter [220]. A definition of failure to control bleeding is stated in five [209, 210, 212, 213, 216]. The efficacy of sclerotherapy for initial hemosta-sis at the end of endoscopy was 95% (76–100%) compared with 97% (86–100%) with ligation EBL group (p = 0.4). In only one [213] of documenting transfusion requirements [211-213, 215, 216, 218], was this less in the ligation group (units, 4.5 ± 1.8 (0–12) versus 3.2 ± 1.2 (0–6) p < 0.01); in others there was no difference. Median quality score was 68.5 (50.5–79). Failure to control of bleeding: there was a difference favoring ligation –2.5% (95% CI: 0.4–4.6%) (p = 0.018). Ala Sensitivity analyses did not show differences from the main metanalysis, including an evaluation defining failure versus those in which there was no failure.
Regarding mortality, the percentage difference was 1.3% favoring ligation (95% CI: –2.3–4.9%, p = 0.46). Sensitivity analyses did not show differences from the main metanalysis.
In another recent trial [221] patients admitted with acute gastrointestinal bleeding and with suspected cirrhosis received somatostatin infusion for five days. Endoscopy was performed within six hours and those with esophageal variceal bleeding were randomized to receive either sclerotherapy (n = 89) or ligation (n = 90). Therapeutic failure occurred in 21 patients treated with sclerotherapy (24%) and in nine treated with ligation (10%) (RR = 2.4, 95% CI: 1.1–4.9). However, in those with active bleeding there was no difference between ligation and sclerotherapy. Failure to control bleeding occurred in 15% vs 4%, respectively (p = 0.02). Treatment group, shock and HVPG > 16mmHg were independent predictors of failure. Side effects occurred in 28% of patients receiving sclerotherapy vs 14% with ligation (RR = 1.9; 95% CI: 1.1–3.5), being serious in 13% vs 4% (p = 0.04). Six-week survival probability without therapeutic failure was better with ligation (p = 0.01) (see Table 36.9 and Figures 36.5 and 36.6).
Figure 36.4 Acute bleeding: Forrest plot of risk difference for mortality in trials of sclerotherapy (S) vs sclerotherapy combined with vasoactive agents (S + D).
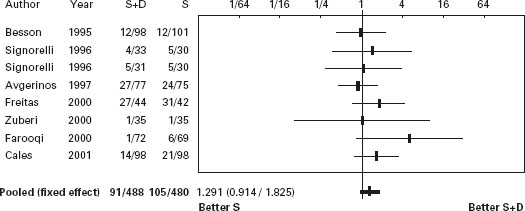
Table 36.9 Randomized controlled trials of sclerotherapy versus variceal ligation for the prevention of re-bleeding.
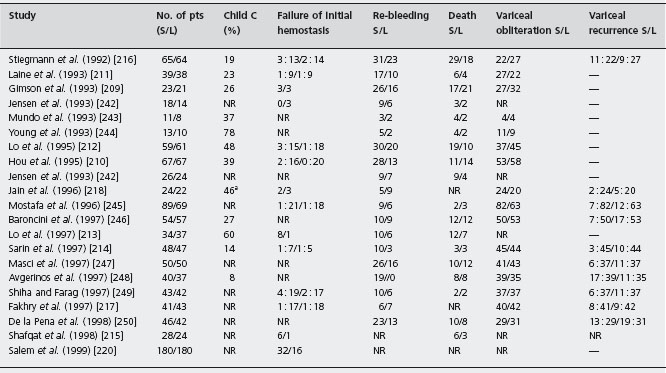
S: sclerotherapy; L: variceal ligation; NR: not reported.
Figure 36.5 Forrest plot of risk difference for control of bleeding in trials of sclerotherapy vs variceal ligation.
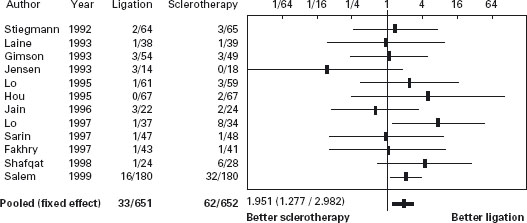
Figure 36.6 Forrest plot of risk difference for mortality in trials of sclerotherapy vs variceal ligation.
Ligation plus sclerotherapy vs ligation
There were 113 patients with either active bleeding or stigmata of recent bleeding from esophageal varices who were randomly assigned to receive ligation plus sclerotherapy, or ligation alone. Hemostasis of active bleeding was achieved at the time of the index treatment in eight of ten of the patients in the combined group (80%) and in ten of twelve of the patients in the ligation group (83%, p>0.05) [222].
Ligation + terlipressin vs ligation + octreotide
In a recent randomized trial [223] 88 patients with cirrhosis with variceal bleeding allocated to terlipressin (n = 43) or octreotide (n = 45) succesfull hemostasis was achieved in 98% of patients. Early re-bleeding, which included re-bleeding within six weeks of therapy was seen in 28% of terlipressin group and 24% of octreotide group. The two treatments were equally effective for acute esophageal variceal bleeding with regard to hemostatic effect and prevention of re-bleeding.
Randomized controlled trials of emergency surgery in the management of acute variceal bleeding
Four randomized trials, carried out in the 1980s, compared sclerotherapy to emergency staple transaction [224–226]. Failure to control bleeding was reported only in two of these studies, with divergent results. Teres et al. reported that efficacy of transection in their study was only 71%, the lowest in the literature, compared with 83% in the sclerotherapy arm [226]. In contrast, in the largest study by Burroughs et al., a five-day bleeding-free interval was achieved in 90% of the patients who underwent transection (none re-bled from varices) compared with 80% in those who had two emergency injection sessions [227]. There was no difference in mortality between the two treatment modalities. Cello et al. showed that emergency portacaval shunt was more effective than emergency sclerotherapy (followed by elective sclerotherapy) in preventing early re-bleeding (19% vs 50%) [228]. Hospital and 30-day mortality were not significantly different. Alc Finally, Orloff et al. in a small study, reported that portacaval shunt, carried out in less than eight hours from admission, was significantly better than medical treatment (vasopressin/ balloon tamponade) in the control of acute variceal bleeding [229]. Survival was also better in the patients who had shunts, but the difference was not statistically significant.
Randomized controlled trials of other endoscopic therapies in the management of acute variceal bleeding
Two types of tissue adhesives (n-butyl-2-cyanoacrylate, Histoacryl and isobutyl-2-cyanoacrylate, Bucrylate) have been used for the control of variceal bleeding [230]. The adhesives potentially result in better immediate control of bleeding because they harden within seconds upon contact with blood. However, extra care must be taken to ensure that the adhesive does not come into contact with the endo-scope and block suction and operator channels. This can be prevented if the adhesive is mixed with lipiodol to delay hardening. Moreover, the sclerotherapy needle must be carefully placed within the varix prior to injection, to avoid leak. A small randomized trial showed that cyanoacrylate was superior to conventional sclerotherapy with 3% eth-anolamine oleate solution for control of bleeding and reduction of hospital mortality in Child-Pugh class C patients [231]. Two randomized controlled trials compared sclerotherapy alone with the combination of sclerotherapy and n-butyl-2-cyanoacrylate [232, 233]. The combined treatment was more effective than sclerotherapy alone in both studies. Alc Moreover, in two studies n-butyl-2-cy-anoacrylate was compared with variceal ligation for the control of bleeding from esophageal [234] or esophagogas-tric varices [235]. The overall success rate for initial hemos-tasis of both treatment modalities was similar in these studies. Alc However, n-butyl-2-cyanoacrylate was superior to variceal ligation for the control of fundal variceal bleeding, but it was less effective for the prevention of re-bleeding (67% vs 28%). In a small randomized study [236], a biological fibrin glue (Tissucol) was more effective than sclerotherapy with polidocanol in the prevention of early re-bleeding and had a significantly lower incidence of complications. Finally, another study found that fibrin glue was as effective and safe as ligation for acute bleeding and for prevention of re-bleeding from esophageal varices, but had superior tissue compatibility [237]. More studies are necessary to confirm these data and examine the potential risks of activating coagulation, systemic embolism and particularly transmission of infections with the human plasma-derived fibrin glue. The literature available on thrombin is limited. Bovine thrombin was effective in small series, but has been withdrawn due to its infection risk [238].
In addition, in a prospective randomized controlled trial [239], cirrhotic patients with acute or recent esophageal variceal bleeding were assigned randomly to percutaneous transhepatic varices embolization, PTVE (52 patients) or EVL (50 patients). During the follow-up period (median 24 and 25 months in the PTVE and EVL groups respectively) UGI re-bleeding developed in eight patients in the PTVE group and 21 patients in EVL group (p = 0.004). Recurrent bleeding from esophageal varices occurred in three patients in the PTVE group and 12 in the EVL group (p = 0.012, relative risk 0.24, 95% CI: l 0.05–0.74). Survival in these two groups was not significantly different.
An endoscopic detachable snare is another ligation device which has the advantage of allowing an unlimited number of ligations by reloading the nylon minisnare while the endoscope remains in the esophagus. The first prospective randomized trial showed that the mini snare performed equally well when compared with a multiple variceal ligator [240]. Ald In another study 50 patients with acute esophageal variceal bleeding were recruited: 25 were treated by elastic band ligation and 25 by endoloop ligation: re-bleeding 12% endoloop group and 28% ligation was not significantly different [241].
Gastric varices
The incidence of bleeding from gastric varices varies between 3% and 30%, but in most series it is less than 10% [251]. Patients with gastric variceal hemorrhage bleed more profusely and require more transfusions than patients with esophageal variceal bleeding [252]. B4 Moreover, these patients have a higher risk of re-bleeding and a decreased survival rate compared with patients bleeding from esophageal varices. The optimal treatment of gastric variceal bleeding is not known. Limited information is available on the role of vasoactive drugs in the control of gastric fundal bleeding and balloon tamponade has been used with little success. Use of standard sclerosants is associated with unacceptable re-bleeding, particularly from necrotic ulceration, as the gastric mucosa appears much more sensitive to this than the esophagus.
Because of this, alternative sclerosant agents have been evaluated. The tissue adhesives n-butyl-2-cyanoacrylate and isobutyl-2-cyanoacrylate, mixed with lipiodol to delay premature hardening, have been evaluated, and found to be efficacious in observational, studies [253]. Isobutyl-2-cyanoacrylate has been shown to be superior to eth-anolamine, in a non-randomized study, achieving hemostasis in 90% of 23 patients, as opposed to 67% of 24 patients (p < 0.005) [254].
In a randomized controlled trial of 37 patients with isolated fundic varices (acute and recent bleeding) with follow-up of 15 months, cyanoacrylate glue was shown to be more effective than sclerotherapy with alcohol for variceal obliteration (100% vs 44%, p < 0.005) [255]. There was a trend in favor of cyanoacrylate glue for the control of acute bleeding (89% vs 62%) and the need of surgical intervention (10% vs 35%), although both were statistically nonsignificant. Mortality was similar in the two groups. Ald
In another randomized trial [256] 48 patients received endoscopic band ligation (GVL) and another 49 patients received endoscopic n-butyl-2-cyanoacrylate injection (GVO). Both treatments were equally successful in controlling active bleeding (14/15 vs 14/15, p = 1.000). More of the patients who underwent GVL had GV re-bleeding (GVL vs GVO, 21/48 vs 11/49; p = 0.044). The two-year and three-year cumulative rate of GV re-bleeding were 63.1% and 72.3% for GVL, and 26.8% for both periods with GVO; p = .0143, log-rank test. Multivariate Cox regression indicated that concomitance with HCC (relative hazard: 2.453, 95% CI: 1.036–5.806, p = 0.041) and the treatment method (GVL vs GVO, relative hazard: 2.660, 95% CI: 1.167–6.061, p = 0.020) were independent factors predictive of GV re-bleeding. There was no difference in survival between the two groups.
In the study by Lo et al. cirrhotic patients with a history of gastric variceal bleeding were randomized to two groups [257]. The group receiving endoscopic obturation (group A) comprised 31 patients and the group receiving band ligation (group B) comprised 29 patients. Butyl cyanoacrylate and pneumatic-driven ligator were applied, respectively. Active bleeding occurred in 15 patients in group A and 11 patients in group B. Initial hemostatic rate was 87% in group A and 45% in group B (p = 0.03). The sessions required to achieve variceal obliteration and obliteration rates were similar in both the groups. However, re-bleeding rates were significantly higher in group B (54%) than group A (31%) (p = 0.0005). Nine patients of group A and 14 patients of group B died (p = 0.05). Authors concluded that endoscopic obturation using cyanoacrylate proved more effective and safer than band ligation in the management of bleeding gastric varices.
However, reports of cerebral embolism, with tissue adhesives identified at postmortem as well splenic emboli-zation and development of retrogastric abscesses, cause concern. Interest has therefore focused on thrombin. This is much easier to administer, and has been shown to provide good early hemostasis [258]. However, in all of these studies, re-bleeding rates have remained high. Hence, in patients with re-bleeding or uncontrolled bleeding from gastric varices, devascularization surgery or portosystemic shunting has been proposed. “Salvage” TIPS is very effective in this situation, with more than 95% success rate for initial hemostasis and an early re-bleeding rate of less than 20% [251]. TIPS appears to be as effective for gastric varices as for esophageal varices.
Non-actively bleeding patients with fundal varices constitute a discrete population. The efficacy of cyanoacrylate in these patients is controversial and bleeding rates in this group can be relatively high. The Japanese experience with balloon-occluded transvenous obliteration as a prophylactic procedure in this patient population appears promising [259, 260]. TIPS, shunt surgery and, of course, liver transplantation are the only other therapeutic options for recurrent bleeding from gastric varices. The consensus at Baveno IV was to use endoscopic therapy with tissue adhesive (e.g. N-butyl-cyanocrylate) [89]. Lastly, in a recent trial TIPS proved more effective than glue injection in preventing re-bleeding from gastric varices, with similar survival and frequency of complications [261].
Uncontrolled variceal bleeding
The definition of uncontrolled variceal bleeding includes the continued/early variceal re-bleeding (within five days) despite two sessions of therapeutic endoscopy, continued variceal bleeding despite balloon tamponade and continued/early gastric or ectopic variceal bleeding despite vasoconstrictor therapy.
Predictive models indicate grade C patients with signs of significant bleeding are the patients at risk of failure. Balloon tamponade has most often been used to arrest life-threatening hemorrhage or if other measures fail. It has also been used in the absence of a definite diagnosis if bleeding from varices is strongly suspected. The usual tube is a modified four-lumen Sengstaken-Blakemore tube (SBT). The airway should be protected by an endotracheal tube under a short general anesthetic, as the risk of aspiration is very high, particularly in unskilled hands [262]. If blood is still coming up the gastric aspiration lumen, then varices are less likely to be the cause of blood loss, although gastric fundal varices are not always controlled by tamponade. In fact, whenever this occurs, if the position of the SBT has been checked and adequate traction applied, the diagnosis of variceal bleeding should be questioned, and emergency angiography performed.
In a recent report the use of self-expandable metallic stents was evaluated to arrest uncontrollable acute variceal bleeding (20 patients mean age 52, 8 Child pugh C) [112]. The patients had not been successfully managed with prior pharmacologic or endoscopic therapy. The stents were successfully placed in all of the patients and were left in place for 2–14 days. Bleeding from the esophageal varices ceased immediately after implantation of the stent in all cases. No recurrent bleeding, morbidity, or mortality occurred during treatment with the esophageal stent. All of the stents were extracted without any complications after definitive treatment had been started.
Randomized trials of TIPS
TIPS stops bleeding in a significant percentage of patients [263]. In uncontrolled studies TIPS is effective in stopping variceal hemorrhage [251, 264, 265], but there is still a high mortality [266]. Monescillo et al. evaluated variceal bleeders with HVPG ≥ 20mmHg randomly allocated to receiving transjugular intrahepatic portosystemic shunt (TIPS; HR-TIPS group, n = 26) within the first 24 hours after admission or not (HR-non-TIPS group) [96]. The HR-non-TIPS group had more treatment failures (p = 0.0001). Early TIPS placement reduced treatment failure (p = 0.003), in-hospital and one-year mortality (p < 0.05). Overall TIPS remains a good choice as a rescue therapy although when it is not available staple transection of the esophagus could be considered [267]. Ald In another multicentre trial an early decision for PTFE-TIPS improved survival in high risk cirrhotic patients admitted with acute variceal bleeding [268].
New diagnostic and treatment algorithms of acute variceal bleeding are needed using known predictive factors of failure to control bleeding and mortality in order to identify the group of bleeders with poor outcome. In this group more effective vasoactive regimens, early TIPS after diagnostic endoscopy, and the use of self-expanding covered esophageal stents could be considered [269].
Conclusion
The available data suggest that emergency endoscopic treatment with banding ligation or sclerotherapy, at the time of the initial diagnostic endoscopy, should be the gold standard for the management of the acute variceal bleeding episode. Sclerotherapy may be more applicable in some acute situations compared with ligation. A diagnostic endoscopy, with visualization unhindered by the ligation device, should be done first as varices may not be the source of bleeding. If ligation is to be used then this entails a double intubation and does lengthen the procedure.
Sclerotherapy is also significantly better than drug treatment alone and there is no need for further studies directly comparing sclerotherapy or ligation with one of the currently available drugs. However, the combination of sclerotherapy with a drug, given as soon as possible after admission is currently the best option. The drugs of choice for this combination are terlipressin (as mortality is reduced albeit in small placebo-controlled studies) and somatostatin (which has less side effects and has been successfully tested over five days). Further studies are needed to assess the role of tissue adhesives or fibrin glues in patients’ unresponsive to vasoactive drugs or sclerotherapy.
The role of emergency TIPS as “salvage therapy” for uncontrolled bleeding from esophageal or gastric varices is now common practice but only certain centres can offer an emergency service. Randomized trials with other therapies such as esophageal stent should be performed.
Prevention of recurrent variceal bleeding
Patients surviving the first episode of variceal bleeding are at very high risk of recurrent bleeding (70% or more) and death (30–50%). All patients who have previously bled from varices should have secondary therapy to prevent further variceal bleeding [89]. There is no role for an observational policy, as all randomized studies have shown active therapy to be better than observation alone. Ala Hence, in clinical practice the risk indicators of long-term re-bleeding are of less clinical value, than those for first variceal bleeding. However, severity of liver disease, continued alcohol abuse and variceal size have been associated with variceal re-bleeding.
A recent development has been the proposed use of hemodynamic indices to identify patients who are more likely to re-bleed. Two such indices have been reported, using the technique of hepatic venous pressure measurement as an indicator of portal pressure. From the analysis of the Barcelona-Boston-New Haven Primary Prophylaxis trial, it was concluded that variceal bleeding did not occur with an HVPG < 12 mmHg, in patients with predominantly sinusoidal portal hypertension. However, with an HVPG > 12 mmHg, the correlation between portal pressure and bleeding risk is inconsistent.
An hemodynamic index was proposed by Feu et al. [270]. Patients who had a percentage reduction of HVPG of 20% or more from baseline had a re-bleeding rate of 15% compared with 50% in those who did not achieve this hemodynamic target. Unfortunately, these HVPG targets are achieved in only about one-third of patients on β-blockers, hence the introduction of combined pharmacologic therapy. Several studies on secondary prevention have used hemodynamic monitoring during combination pharmacother-apy in their design. In these studies the target values are reached only in 45–60% of patients subjected to therapy with β-blockers and nitrates. Moreover, because most re-bleeding episodes occur within one month of the index bleed, early repeat HVPG measurements are needed in order to identify the nonresponders. However, the above approach has not been confirmed in one study [271]. A randomized study has shown greater likelihood of HVPG reduction with sequential monitoring of portal pressure but no reduction in rebleeding compared to combined ligation and nadolol without monitoring of HVPG [272].
There are several options including pharmacologic, endoscopic and surgical/radiological therapies.
Randomized controlled trials for the prevention of variceal re-bleeding
β-blockers versus no treatment
A comprehensive meta-analysis of 12 trials comprising 769 patients [273–284] has been published [285]. The mean follow-up was 21 ± 5 months. There was significant heterogeneity in the evaluation of re-bleeding (p < 0.01). Treatment with β-blockers significantly decreased the risk of re-bleeding (Der Simonian and Laird method: POR 21%; 95% CI: 10–32%). The NNT with β-blockers to prevent one re-bleeding episode was 5. Survival was also significantly improved in patients treated with β-blockers. (Der Simonian and Laird method: POR 5.4%, 95% CI: 0–11%) although there was significant heterogeneity in this analysis (p < 0.01). Ala The NNT to prevent one death is 14. Adverse events occurred in 17% of patients and were generally mild. No fatal complication has been reported with β-blockers.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







