+ Superior to control treatment; = equal to control treatment; ± conflicting results; nd: not done.
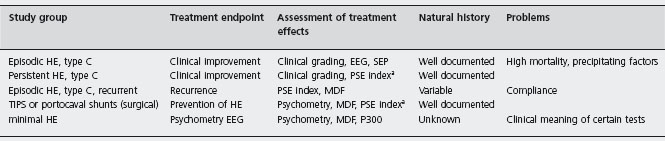
Methods to quantify
Clinical assessment
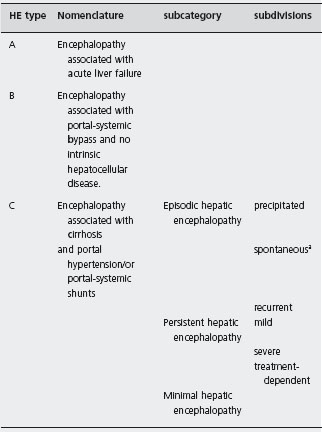
The simplest assessment of HE is a description of the mental state, according to Conn [12], which grades HE in stages I–IV based on changes in consciousness, intellectual function and behavior. It does not include neurological changes or asterixis. The Glasgow Coma scale is useful in stages III and IV.
The PSE (portal–systemic encephalopathy) index
In 1977 the “PSE index” was introduced in a trial comparing neomycin with lactulose and has been subsequently used by other investigators [13]. The main problem with this index is the inclusion of arterial ammonia estimations. Hyperammonemia is possibly a cause, but not a symptom or effect of HE. Measurements of arterial ammonia concentrations require serial arterial punctures. The scoring of actual arterial ammonia concentrations is arbitrary and not based on a sound statistical analysis. Furthermore, the other parameters of the PSE index – mental state, EEG and number connection tests (NCT) – are also graded by arbitrary units. No age-dependent normal values are used for NCT [14]. Finally, the PSE index does not discriminate between overt, mild or subclinical HE and has not been validated prospectively. In clinically overt HE the PSE index does not appear to be superior to simple clinical grading.
aWithout recognized precipitating factors.
Psychometric tests
Grading of HE does not allow the documentation of subtle changes. To quantify the impairment of mental function in mild stages of HE several psychometric tests have been evaluated [15, 16, 17]. Detailed psychometric testing is more sensitive in the detection of minor deficits of mental function than either conventional clinical assessment or the EEG [16]. However, the tests are cumbersome, and when applied repeatedly the reliability of most of them is adversely affected by the learning effect. Few are useful in routine practice. The most frequently applied test is the number connection test [14, 16]. This test is easily administered and the results can be quickly quantified. One important consequence of the application of psychometric tests in cirrhotic patients was the finding that even patients with apparently normal mental status have a measurable deficit in their intellectual performance [13]. These patients are usually referred to as suffering from “minimal HE” or “stage 0 HE”. However, psychometric tests may overdiag-nose minimal HE, because scores are usually not corrected for age [14,18]. Furthermore, it is unknown whether abnormalities of test results correlate with impaired quality of life or performance in daily life [18]. On the contrary, the driving ability of patients with test results classifying them as “unable to drive a car” [15] was not different from that of healthy controls. A quality-of-life questionnaire (sickness impact profile (SIP)) detects the extent and frequency of deficits in daily functioning in patients without clinically apparent HE. From the 136 statements, five were selected as predictive of minimal HE [18].
a PSE index according to Conn et al. [2] (the use of this index was not recommended by the WCOG-Working party 1a).
SEP: somatosensory evoked potentials; MDF: mean dominant frequency; P300: event related acoustic evoked responses.
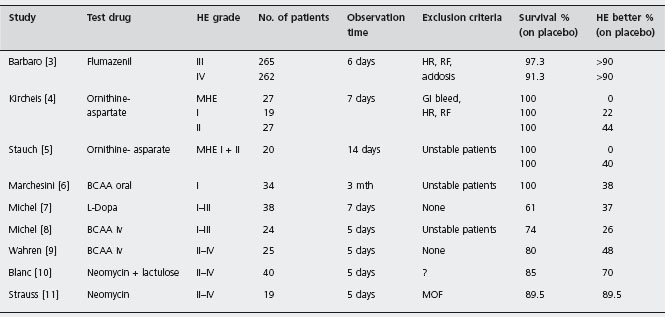
Table 39.4 Survival rates and improvement of hepatic encephalopathy in placebo treated patients in randomized controlled trials.
aAll patients were on neomycin.
HR, hepatorenal syndrome; RF, respiratory failure; MOF, multiorgan failure; BCAA, branched chain amino acids; MHE, minimal hepatic encephalopathy.
A standardized prospectively developed test battery that includes the NCT A and B, the line tracing test, the serial dotting test and the digit symbol test was recommended by the working party to be used in future studies [2]. This test (PHES) can be applied at the bedside and performed within 10–20 minutes and examines visual perception, visuo-spatial orientation, visual construction, motor speed and accuracy, and is also sensitive against disturbances of concentration, attention and working memory. Each individual test and the whole battery has been standardized in a large group of healthy controls (including all ages). A composite score of the single test results was calculated. Each of the individual test results was scored 0 points in the ± 1 SD range from the mean. Thereby, subjects can achieve between +6 and –18 points. When a cutoff between normal and pathological results was set at –4 points, only 1 (0.9%) of the controls, 25% of cirrhotic patients without clinical evidence of HE, but all patients with grade I HE achieved pathological results. B2 The test has a high specificity for HE as compared with other metabolic encepha-lopathies [19,20].
Electrophysiological tests
The simplest EEG assessment of HE is to grade the degree of abnormality of the conventional EEG trace. A more refined assessment by computer assisted techniques allows variables in the EEG such as the mean dominant EEG frequency and the power of a particular EEG rhythm to be quantified. Evoked responses (by visual, somatosensory, or acoustic stimuli) or event related responses, like the P300 peak after auditory stimuli, are sensitive to detect subtle changes of brain function and can be used for diagnosis of minimal HE [20].
Critical flicker frequency
Retinal glial cells are involved in ammonia detoxification by glutamine synthesis. In patients with liver failure, they exhibit morphological changes similar to those observed in brain astrocytes, suggesting that retinal gliopathy could serve as a marker of cerebral gliopathy in patients with hepatic encephalopathy. These observations provided the rationale for the development of a visual test (the critical flicker/fusion frequency) for determining whether hepatic encephalopathy is present. Initial experience suggests that the critical flicker/fusion frequency may be a highly objective and sensitive measure of minimal hepatic encephalopathy [21, 22, 23].
Diagnosis of minimal hepatic encephalopathy
One important consequence of the application of psychometric tests in patients with cirrhosis was the finding that even patients with apparently normal mental status have some form of a measurable deficit in their intellectual performance, long-term memory, and learning capability [15, 24]. These patients are usually referred to as “minimal HE” or “stage 0 HE”. Up to 15% of patients with cirrhosis have minimal HE when defined by the presence of a prolonged NCT or an abnormal EEG [25]. Several approaches are being studied to evaluate for minimal hepatic encephalopathy. At present, none is used routinely in clinical practice.
One study comparing the Psychometric Hepatic Encephalopathy Score (PHES) with an EEG in 100 patients with cirrhosis found agreement in detection of MHE in only 73% [26]. The poor correlation may reflect differences in how these tests detect various features of MHE.
The Inhibitory Control Test (ICT) is a computerized test of attention and response inhibition that has been used to characterize attention deficit disorder, schizophrenia and traumatic brain injury. The subject is instructed only to respond to two alternating letters (X/Y) (called “targets”) and not to respond when they are not alternating (called “lures”). Lower lure responses, higher target responses, and shorter lure and target reaction times indicate good psychometric performance. A study comparing ICT to a psychometric battery of tests for MHE diagnosis in 136 patients estimated sensitivity for MHE to be 88% [27]. Patients with MHE had significantly higher ICT lures and lower targets compared to patients without MHE.
The PHES was compared with a comprehensive computerized assessment (Cognitive Drug Research [CDR]) of cognitive function in 89 patients with cirrhosis [28]. There was a high correlation between the two assessment methods. The MELD score correlated with PHES. In contrast, the CDR domains Continuity of Attention and Quality of Episodic Memory were significantly related to venous blood ammonia levels. There were marked deteriorations in the CDR composite scores representing Accuracy of Working and Episodic Memory after amino acid challenge to increase blood ammonia. Both PHES and CDRS returned to the control range after liver transplantation.
The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) measures a wide range of neurocognitive functions relevant to MHE. The test has been used in multiple clinical trials in the USA for a variety of neurologic disorders and in patients with advanced cirrhosis [29]. The RBANS has not yet been compared directly with the PHES, and its responsiveness to hepatic encephalopathy treatment is unknown.
A concern in patients with minimal hepatic encephalopathy is whether they are at increased risk for driving accidents. Studies evaluating this question have produced disparate conclusions. At least three reports concluded that 44–60% of patients with advanced liver disease (but without overt clinical signs of HE) were unfit to drive based upon the results of extensive batteries of neuropsychologic tests [1, 21, 22]. In one study, for example, 40 patients with chronic liver disease and portal hypertension without clinical signs of portasystemic encephalopathy were given the same extensive psychometric tests typically used for expert reports on driving capacity [15]. Sixty percent of patients were considered to be unfit to drive, and 25% were considered to be questionable. The total driving score of patients with minimal hepatic encephalopathy was significantly reduced compared with controls or cirrhotic patients without minimal hepatic encephalopathy [30]. A later report found that patients with minimal hepatic encephalopathy had poor insight into their driving skills [31].
Evidence-based medicine and hepatic encephalopathy
Evidence based medicine is a process of systematically finding, appraising, and using research findings as the basis for clinical decisions [32] based on the formulation of relevant questions concerning a patient’s problem.
The answer to the question “Does treatment with specific drugs, compared to placebo, improve HE?” should be addressed separately for overt and subclinical HE. In the following sections we have identified the studies that attempt to answer this question and we have critically appraised the evidence for the most important treatment regimens. The magnitude of the treatment effect of various interventions has been assessed. This assessment is difficult in HE because of the use of different methods which are not readily comparable for quantifying the severity of this disease. The question of the clinical applicability and generalizability of the findings of randomized controlled studies in HE must be addressed in the context of the treatment and the grade of encephalopathy studied.
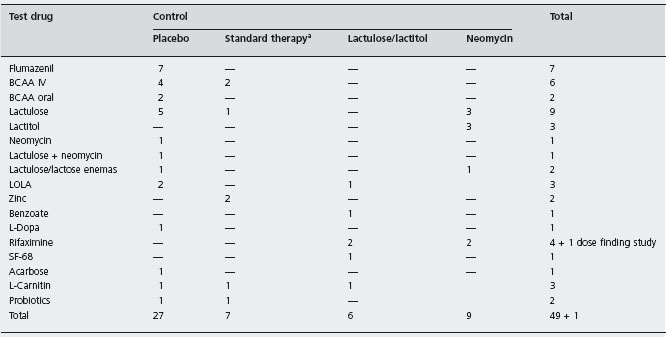
To identify all randomized controlled trials in HE, a Medline search was conducted using several terms. A total of 50 randomized trials had the endpoint “improvement of HE” and included more than ten patients per study group (see Table 39.5). In addition, two meta-analyses have been published [33, 34].
Treatment of hepatic encephalopathy
Clinically overt he (grade I–IV) in patients with cirrhosis
Supportive care and treatment of precipitating causes of HE
It is important to recognize that HE, acute and chronic, is reversible and that a precipitating cause rather than worsening of hepatocellular function can be identified in the majority of patients [1, 2].These causes include gastrointestinal bleeding, increased protein intake, hypokalemic alka-losis, infection, and constipation (all of which increase arterial ammonia levels), hypoxia, and the use of sedatives and tranquilizers. Patients with advanced cirrhosis may be particularly sensitive to benzodiazepines.
Treatment of these precipitating events is typically associated with a prompt and permanent improvement of HE. As a result, every attempt should be made to identify and to treat such precipitating events. This approach has never been tested formally but is based on common clinical experience. As judged from the outcomes observed in placebo groups of controlled studies (see Table 39.3) standard medical care is highly effective.
Enemas
Cleansing of the colon by enemas is a rapid and effective procedure to remove ammoniagenic substrates. The efficacy of enemas of 1–3 liters of 20% lactulose or lactitol solutions was proven in randomized controlled trials; a favorable response was noted in 78–86% of patients (ARR 0.4%, NNT = 2.5) [35, 36]. A1d Interestingly, enemas with tap water were ineffective, raising the possibility that colonic acidification rather than bowel cleansing was the effective therapeutic mechanism.
Nutrition
Patients with grade III–IV HE usually do not receive oral nutrition. In general, there is no need for parenteral nutrition if patients improve within two days.
Based on the “false neurotransmitter hypothesis”, total parenteral nutrition with specific amino acid solutions has been proposed. A number of randomized controlled studies have evaluated the use of solutions with a high content of branched chain amino acids (BCAA) and a low content of aromatic amino acids (AAA). These studies differ with respect to the amino acid solutions used, the study protocols, patient selection and the duration of treatment, and are difficult to compare. The results have been conflicting, but most studies did not find any improvement in HE or any reduction in mortality in patients treated with BCAA [37, 38]. Although a meta-analysis revealed a significant trend toward improvement in these outcomes, it was concluded that further randomized controlled trials are needed [34]. At present, infusions of modified amino acid solutions should not be used in the standard treatment of patients with HE. A1c
There is no proven need for a specific diet for patients with HE. Although mentioned in all textbooks, the recommendation of a low protein diet in patients with advanced liver disease is not supported by good clinical or experimental evidence. On the contrary, in patients with alcoholic hepatitis, low protein intake is associated with worsening HE, while a higher protein intake correlates with improvement in HE [39]. The recommendations of the European Society of Parenteral and Enteral Nutrition (ESPEN) are that oral protein intake should not exceed 70g/day in a patient with a history of HE; a level below 70g/day is rarely necessary and minimum intake should not be lower than 40g/day to avoid negative nitrogen balance [40]. C5
a Usually includes lactulose or neomycin.
Pharmacotherapy
Flumazenil
Based upon the GABA-benzodiazepine hypothesis of the pathogenesis of HE, the benzodiazepine receptor antagonist flumazenil has been tested for treatment of HE in five randomized placebo controlled trials involving over 600 patients. Four were crossover trials, and one used a parallel group design. Flumazenil was superior to placebo in four of these studies (Table 39.5). In the only large double-blind, placebo controlled crossover trial 537 cirrhotic patients with grade III (265 patients) or IVa (262 patients) hepatic encephalopathy were randomized to receive intravenous flumazenil or a placebo over a 3–5 minute period [3]. Patients subsequently received the other study medication if they were still in grade III or IVa encephalopathy after the first study period. Treatment was begun within 15 minutes of randomization. Outcome measures included both a neurological score and a grading derived from continuous EEG recordings. Table 39.6 shows the results obtained by combining the scores from the initial and crossover period. Improvement of the neurological score was documented in 46 of grade III and in 39 of grade IVa patients during the combined flumazenil treatment periods and in 10 (Grade III) and 3 (Grade IVa) of the patients during placebo treatment periods. Improvement of the EEG score occurred in 73 (Grade III) and 57 (Grade IVa) patients during flumazenil treatment and 13 (Grade III) and 9 (Grade IVa) patients during placebo treatment. The effects of flumazenil were statistically significant (p < 0.01). A1d In the second study [41], 24 of 49 randomized patients were excluded from the final analysis, mainly due to inadequate benzodiazepine screening. However, flumazenil was superior to placebo even when the data were evaluated by intention-to-treat analysis; among the 25 patients who were not excluded, clinically relevant improvement was seen in 35% compared to 0% in patients given placebo. A1d In the Canadian trial, very strict exclusion criteria resulted in the rejection of 56 of 77
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree