Fig. 36.1
Endoscopic appearance of a pedunculated adenoma
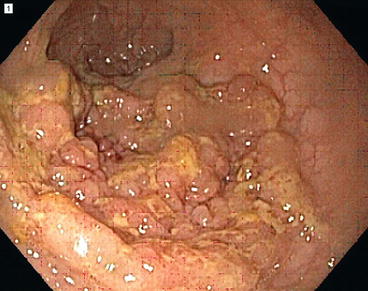
Fig. 36.2
Endoscopic appearance of a sessile adenoma (Courtesy of Roberta L. Muldoon)
Adenomas are classified as tubular, villous, or tubulovillous. The former lesions comprise approximately 75–87 % of all adenomas identified in the colon and contain uniform-sized tubules and glands. As the tubules become more elongated with less stroma between glands, they assume a more villous character. For pathologists, tubular adenomas (Fig. 36.3) can consist of up to 20–25 % villous features and still be considered a “tubular adenoma,” while villous adenomas (5–10 % of all adenomas) contain more than 50–75 % villous features, making tubulovillous adenomas (8–15 % of all adenomas, Fig. 36.4) those polyps in between. While the likelihood of a polyp to harbor malignancy may be impacted by this classification, the treatment for the three classes of adenomas remains the same and thus has little true clinical significance.
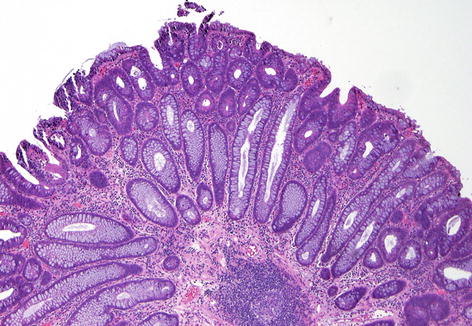
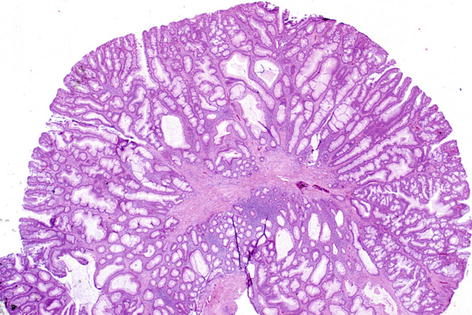

Fig. 36.3
Microscopic view of a tubular adenoma (Courtesy of William Chopp, MD)

Fig. 36.4
Microscopic view of a tubulovillous adenoma (Courtesy of M. Kay Washington, MD, PhD)
Adenomas are differentiated from hyperplastic polyps in that they display cellular atypia with lack of differentiation into specialized cell types. The epithelial lining will show increasing mitoses and some degree of hyperchromasia (darker hematoxylin and eosin staining) depending on the degree of dysplasia. In adenomas, the normal process of cellular maturation and differentiation from the base of the crypt to the surface does not occur.
Adenomas can be graded by the degree to which epithelial growth is disturbed. Mild or low-grade dysplasia is characterized by tubules, which are lined from top to bottom by epithelium, which is morphologically similar to the normal basal proliferative zone. The nuclei are enlarged, oval, and hyperchromatic and have normal orientation. There is a slight excess of mitotic figures, but the architecture is not disrupted.
By definition, all adenomas show at least low-grade dysplasia. In moderate dysplasia, the nuclear features are more advanced, cellular polarity is less preserved, there is nuclear stratification, and the glands are more crowded. In severe or high-grade dysplasia, there are large vesicular nuclei, irregular and conspicuous nucleoli, scalloped nuclear membranes, and increased nuclear to cytoplasmic ratio. Nuclear polarity is disrupted, and marked cellular pleomorphism and both numerous and aberrant mitoses are present. Structural alterations include budding and branching tubules, back-to-back arrangement of glands, and cribriform growth of epithelial cells in clusters and sheets. The terms “carcinoma in situ” and “intramucosal carcinoma” are often used to describe these high-grade dysplastic adenomas, but these terms are potentially misleading as these lesions do not have metastatic potential.
Presentation and Diagnosis
Most adenomas are asymptomatic and are therefore found with screening studies or incidentally diagnosed through investigations of symptoms unrelated to the adenoma.
Larger adenomas may display overt hematochezia or anemia secondary to occult or overt blood loss. Adenomas in the rectum may cause rectal bleeding, mucoid discharge, tenesmus, and/or fecal urgency. Very large adenomas may rarely cause electrolyte abnormalities or diarrhea or may lead to intussusception of the colon or prolapse through the anus.
Adenomas are often multifocal and can be identified anywhere in the colon and rectum but tend to be more prevalent distally.
In one large prospective study (US National Polyp Study), the distribution of adenomas was as follows: cecum 8 %, ascending colon 9 %, hepatic flexure 5 %, transverse colon 10 %, splenic flexure 4 %, descending colon 14 %, sigmoid 43 %, and rectum 8 %.
Other studies have also documented that 24–31 % of adenomas are proximal to the splenic flexure in colonoscopies in higher-risk or symptomatic patients.
In addition, when a sporadic adenoma is identified in the colon or rectum, the likelihood of a synchronous adenoma elsewhere in the colon ranges from 31 to 40 %. Therefore, when a distal adenoma is found, a complete colonic assessment is necessary because of this high rate of synchronous neoplasms.
Most, but not all, studies of screening flexible sigmoidoscopy suggest that patients with no distal polyps, distal hyperplastic polyps, or a single small tubular adenoma have a low risk of proximal advanced adenomas (0–4 %). Multiple other studies, however, support the recommendation that villous adenomas (regardless of size) and any adenoma >1 cm are important markers for the presence of advanced adenomas and even carcinoma in the proximal colon.
The fact that adenomas are often asymptomatic precursor neoplasms justifies the use of screening to identify and remove these lesions before they become clinically recognizable, thus halting the adenoma to carcinoma sequence (discussed below).
Colonoscopy is the most accurate test for polyps, especially when compared to double-contrast barium enema (DCBE) as shown by the US National Polyp Study.
DCBE alone has been repeatedly shown to be less sensitive than colonoscopy (even for polyps >10 mm with a miss rate of 52 %), offers no therapeutic benefit, and has not been shown to reduce cancer incidence or mortality.
Colonoscopy, on the other hand, decreases the risk of colorectal cancer incidence by 76–90 % and has been indirectly shown to reduce cancer mortality.
Flexible sigmoidoscopy has also been shown to lead to a decrease in distal colon cancer mortality as much as 80 % (45 % for all colorectal cancers) but does not show a reduction in deaths from more proximal cancers.
More recently, CT colonography or “virtual colonoscopy” has been supported as a potential screening modality.
Three meta-analyses (between 1,300 and 6,400 patients in each analysis) have shown sensitivities and specificities for detecting polyps ≥10 mm to be in the 85–95 % and 95–97 % ranges, respectively. Medium-sized polyps (6–9 mm) had lower sensitivities and specificities of 70–86 and 86–93 %, respectively.
Newer screening modalities such as chromoendoscopy or dye-spray endoscopy, narrowband imaging, magnification endoscopy, and pill colonoscopy have not been established as effective means for surveillance or screening for all patients and are not equivalent in the hands of all providers.
Epidemiology
Colonoscopy-determined prevalence rates in asymptomatic, average-risk individuals ≥50 years range from 24 to 50 %, with the prevalence of advanced adenomas (≥1 cm in size, with villous features, and/or with high-grade dysplasia) varying from 3.4 to 9.5 % depending on age and gender.
Prevalence rates have been shown to increase with age, even doubling between ages 50 and 60.
Higher adenoma prevalence rates have been identified in men, with a relative risk of 1.5–2.0 compared to age-matched women.
Interestingly, however, in one study of screening colonoscopies performed on 1,463 asymptomatic women ≥40 years old, 20.4 % were diagnosed with an adenoma and 4.9 % with an advanced adenoma. When these women were compared to a matched group of men (8.6 % of whom had advanced adenomas on screening colonoscopy), almost 65 % had their advanced adenomas in the proximal colon versus 34 % of the men, suggesting that gender differences may lead to changes in adenoma location as well as overall prevalence.
In terms of family history risk, a multicenter screening colonoscopy study examining the risk of colorectal adenomas in a cohort of individuals with one affected first-degree relative with sporadic colorectal cancer found the odds ratio to be 1.5 for adenomas, 2.5 for large adenomas, 1.2 for small adenomas, and 2.6 for high-risk adenomas (see below).
The prevalence of adenomas and advanced adenomas is higher in relatives of individuals with colorectal cancer or adenoma at a young age and in individuals with multiple relatives with cancer or adenomas.
Adenoma prevalence rates determined by colonoscopy are roughly double the rates determined by flexible sigmoidoscopy.
The prevalence of a proximal synchronous adenoma in a patient with a distal adenoma (or even hyperplastic polyps in some studies) is such that proximal colonic assessment is warranted if a distal lesion is found on screening.
The incidence of adenomas is the rate at which individuals develop colorectal adenomas over a specified time interval.
The incidence of adenomas at intervals ranging from 6 months to 5 years in post-polypectomy surveillance colonoscopy studies varies from 20 to 50 %.
Most incident polyps are small, and a higher incidence has been associated with multiple adenomas at the index colonoscopy, larger size of the index adenoma, older age, and a family history of a parent with colorectal cancer.
The incidence rate of colorectal adenomas after a clearing colonoscopy is actually the sum of the true incidence rate of new adenoma formation plus the miss rate at the initial colonoscopy plus the recurrence rate of incompletely removed polyps.
Judging by repeat endoscopy, including studies with same day back-to-back colonoscopies, the miss rate for adenomas ≥1 cm is approximately 5 %, for adenomas 6–9 mm it is approximately 10 %, and for adenomas ≤5 mm it approaches 30 %.
These high miss rates for small lesions suggest that many adenomas detected on surveillance colonoscopy are actually lesions that were missed during the index examination.
Incident polyps are distributed more proximally, consistent with the observation that miss rates for adenomas are higher in the proximal colon.
The incidence rate for advanced adenomas ranges from 6 to 9 % and is closely related to the findings at initial colonoscopy.
Based on a pooled analysis of more than 9,000 patients in North America, of which 11.2 % had advanced neoplasia (adenoma or cancer) on subsequent colonoscopy, a greater number of adenomas at initial colonoscopy, histologic features (villous architecture) of the excised adenoma, larger adenoma size, proximal adenoma location (odds ratio [OR] 1.68; 95 % confidence interval [CI], 1.43–1.98), and male gender (OR 1.40; 95 % CI, 1.19–1.65) were all attributed to increased risk of development of advanced neoplasia.
Three or more polyps at the initial colonoscopy have been shown to increase the risk of subsequent advanced adenomas, and in the US National Polyp Study, age >60 years plus a family history of a parent with colorectal cancer was also a predictor of incident advanced adenomas.
The cumulative incidence of advanced adenomas at 3 and 6 years of follow-up in the US National Polyp Study in the highest-risk group (three or more adenomas at baseline or age ≥60 years plus a parent with colorectal cancer) were 10 and 20 %, respectively.
The lowest-risk group (only one adenoma and age <60 years at baseline) had an incidence of advanced adenomas of <1 % at both 3 and 6 years of follow-up.
The 5-year incidence of advanced adenomas in individuals with a previously negative colonoscopy is also <1 %.
Similarly, post-polypectomy surveillance studies have shown that cancer incidence is also low, and in the US National Polyp Study, colonoscopic surveillance was associated with a 76–90 % reduction in the cancer incidence compared to reference populations.
The rare appearance of incident cancers at short intervals in patients who have had a clearing colonoscopy suggests that either the neoplasm was initially missed or incompletely treated (27–31 % of incident cancers may be due to “ineffective” polypectomy) or the cancer developed rapidly.
Based on long-term follow-up from the Polyp Prevention Trial, these interval cancers are even more common in those patients with a previous history of advanced adenoma.
Despite the initial colonoscopy miss rates, however, modeling shows that >90 % of the reduced incidence of colorectal cancer over the first 5–6 years after screening colonoscopy is the result of the initial polypectomy rather than removal of adenomas at subsequent surveillance.
Adenoma to Carcinoma Sequence
The idea that an adenoma would progress into a carcinoma has been based primarily on observational epidemiologic studies, clinical studies, pathologic findings, and molecular genetic studies, and therefore the evidence, while extensive, is truly circumstantial.
Given the high prevalence of sporadic adenomas in the general population but the relatively low lifetime risk of developing colorectal cancer in Western countries (6 % by age 85), it appears that only a few adenomas become adenocarcinomas.
While not all adenomas develop into colorectal cancer, it appears that most sporadic colorectal cancers (80–85 %) develop from adenomas, although there is evidence for rare de novo colorectal cancer development as well as other less-rare carcinoma sequences.
Adenoma size seems to be important in the likelihood for malignant degeneration, and the likelihood that a diminutive tubular adenoma will progress to become an adenocarcinoma is likely very low.
In a study that analyzed 7,590 adenomatous polyps to determine risk factors for high-grade dysplasia or invasion, size was the strongest predictor. The percent of adenomas with high-grade dysplasia or invasive cancer based on the size of the polyp was as follows: <5 mm, 3.4 %; 5–10 mm, 13.5 %; and >10 mm, 38.5 %. No invasive cancer was found in polyps ≤5 mm. Villous change, left-sided lesions, and age ≥60 years were also associated with advanced histologic features.
One longitudinal study showed that over a 3–5-year period, only 4 % of 213 adenomas measuring 2–15 mm increased in size.
A mathematical model suggested that it takes 2–3 years for an adenoma ≤5 mm to grow to 1 cm and another 2–5 years for the 1 cm adenoma to progress to cancer.
For a lesion ≥1 cm, the cancer probability is 3, 8, and 24 % after 5, 10, and 20 years, respectively.
Overall, the yearly rate of conversion from adenoma to carcinoma has been estimated to be 0.25 %, but the risk is higher depending on size and histologic factors such as the conversion rate for polyps >1 cm (3 %), for villous adenomas (17 %), and for adenomas with high-grade dysplasia (37 %).
Gender does not appear to affect the rate of transition from advanced adenoma to carcinoma, but age clearly impacts malignant degeneration (ranging from 2.6 % at age <60 to >5 % annually at age >80 years for both men and women).
On a molecular level, the “traditional” pathway from adenoma to adenocarcinoma (also known as the “loss of heterozygosity” (LOH) or “chromosomal instability” (CIN) pathway), thought to account for the development of 80–85 % of sporadic colorectal cancers, was elucidated from studies on patients with familial adenomatous polyposis (FAP).
The process starts with a single colorectal epithelial cell undergoing a series of genetic alterations leading to the inactivation of both copies of the tumor suppressor adenomatous polyposis coli (APC) gene on chromosome 5q that regulates cell growth and apoptosis. This appears to occur very early in the process of the normal epithelial cell transitioning into adenomatous tissue or low-grade dysplasia by leading to increased cell proliferation.
The next alteration in the pathway is thought to occur with k–ras, an oncogene involved in signal transduction from the cell membrane to the nucleus. Mutation of this gene (seen in 50 % of colorectal cancers) in the setting of the APC mutation appears to lead to exophytic growth and transition to an “intermediate” adenoma.
Important to the transition from intermediate to advanced adenoma is mutation of the deleted in colon cancer (DCC) gene that is important for encoding an adhesion molecule and facilitating apoptosis and therefore tumor suppression.
The final step to the development of invasive adenocarcinoma (found in 75 % of colorectal adenocarcinomas) is a mutation in the p53 gene, which regulates the cell cycle after DNA injury to allow for DNA repair.
The accumulation of some or all of these molecular abnormalities is therefore associated with the development of invasive colorectal cancer.
The “serrated neoplasia” pathway (see below) is thought to account for the other 10–15 % of sporadic colorectal cancers. This pathway is characterized by cancers showing microsatellite instability (MSI), likely due to hypermethylation of the hMLH1 mismatch repair gene promoter leading to its inactivation, likely occurring after BRAF (a serine–threonine kinase involved in the k–ras pathway) gene mutations. These cancers are morphologically and pathologically similar to the MSI cancers that are associated with the germline mismatch repair gene mutations seen in hereditary nonpolyposis colorectal cancer (HNPCC)/Lynch syndrome (see Chap. 37). While these cancers do appear to develop through an adenoma–carcinoma sequence, the adenomas are not considered the traditional adenomas seen in the APC adenoma–carcinoma sequence and are more likely the SSAs discussed below. See Chap. 38 for a more detailed review on the molecular basis of carcinogenesis.
Management
All adenomas or apparent adenomas should be completely removed for confirmation of the diagnosis and to exclude a concurrent malignancy and the potential need for further intervention.
The majority of adenomas are amenable to endoscopic removal by various means including “cold” (without electrocautery) or “hot” (with electrocautery) biopsy forceps or loops/snares.
Complications of polypectomy, primarily bleeding and perforation, can be limited through the appropriate use of these standard techniques.
Because of the concern for perforations related to the use of cautery, recommendations include limiting the use of hot forceps to small polyps (<5 mm) while tenting the mucosa and somewhat deflating the colon.
The majority of these smaller polyps are usually amenable to single-bite or piecemeal excision with cold forceps that will eliminate the cautery risks.
Large pedunculated polyps can often be removed with snare cautery techniques (although bleeding is uncommon after removal of these). The important aspect of removal of these types of polyps is to ensure that the blood supply through a thick stalk (>1 cm), which may contain substantial vasculature, is controlled prior to the polypectomy. Alternatively, metal clips or endoloops can be placed at the base, or the stalk can be injected with epinephrine to provide hemostasis. The base of the stalk may be tattooed with ink or carbon agents to allow for subsequent identification (endoscopically or surgically) if the polyp has a concerning appearance for malignancy. At times, piecemeal resection of the polyp head is necessary before a large snare can even get around the polyp to reach the stalk.
Larger sessile polyps (>15–20 mm) will usually require piecemeal resection with a large snare cautery. Safe polypectomy while avoiding injury to surrounding normal tissues may also be facilitated by saline lift as described below. When performing a standard piecemeal polypectomy, starting on the proximal aspect of the polyp with or without using a spike-tip snare (allows the snare to be anchored so that pushing the sheath causes the snare loop to widen for more effective placement around the polyp) will allow for easier and more complete polyp resection. While the piecemeal technique is an effective means of removal, it requires meticulous removal of the entire polyp and capture of the pieces. This technique ensures that pathologic examination of the polyp will be complete, although the margins will be unclear when the specimen is resected in this fashion. Larger pieces might require basket retrieval and division of the larger pieces with the cold snare or may necessitate multiple insertions and withdrawals of the colonoscope to remove them.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






