Chapter 21 PHARMACOLOGIC NEUROMODULATION
INTRAVESICAL CAPSAICIN AND RESINIFERATOXIN
Anatomy and Function of the Uroepithelium
The internal surface of the urinary bladder is lined with transitional epithelium. Three distinct layers of the uroepithelium have been described.1 Large umbrella cells form the superficial lining of the bladder urothelium. The permeability barrier of the urothelium is maintained in this stratum by tight junctions that prevent the paracellular passage of urinary solutes across this outer layer into the bloodstream. Umbrella cells are associated with a group of crystalline protein plaques called uroplakins that also contribute to the permeability barrier of the urothelium. Beneath the umbrella cell layer is the inter-mediate layer of moderately sized cells and a basal layer of small cells.2 In the bladder, suburothelial afferent fibers form a dense plexus that innervates both the detrusor itself and the basal layer of the urothelium.3 These afferent fibers are critical for sending sensory input to the central nervous system. The uroepithelium was once thought to serve only as a passive barrier to the contents of the bladder, but there is a growing body of evidence that confirms reciprocal communication between the neuronal system and the uroepithelium. This implies that the uroepithelium may play a significant role in regulating bladder activity via a number of neuron-like properties that modulate bladder sensory function and may represent a potential novel therapeutic target for pharmacologic intervention for bladder dysfunction.
Afferent Innervation of the Urinary Bladder
Normal storage of urine is dependent on spinal reflex mechanisms that activate somatic and sympathetic pathways to the bladder outlet and detrusor muscle as well as tonic inhibitory pathways that suppress parasympathetic excitatory activity leading to detrusor relaxation and bladder filling. Micturition requires efferent nerve input to the bladder from the spinal cord as well as afferent input from the bladder to central nervous system. Sensory information, including the sensations of bladder fullness and pain, is conveyed to the spinal cord via afferent fibers in the pelvic and hypogastric nerves. The most important afferents controlling the micturition process are the small myelinated Aδ fibers, which transmit signals mainly from mechanoreceptors that detect wall tension and bladder fullness, and unmyelinated C fibers, which detect noxious signals and initiate painful sensations.4–6 At other sites in the body, such as the skin and mucous membranes, C-fiber afferents transmit nociceptive information into the central nervous system and modulate various reflex responses to noxious stimuli such as hot temperatures (see later discussion).
After suprasacral spinal cord injury, there is significant reorganization of micturition reflexes that leads to the emergence of primitive spinal bladder reflexes triggered by C-afferent neurons. Afferent C-fiber activation may also occur in infectious or irritative conditions in the bladder and serve to facilitate or trigger voiding. This may be viewed as a defense mechanism to eliminate bladder irritants or bacteria. Bladder wall C fibers may be responsible for afferent signals that trigger detrusor overactivity (DO). As specific C-fiber neurotoxins, capsaicin and resiniferatoxin (RTX), its ultrapotent analogue, may be used to study and treat lower urinary tract dysfunction.7,8
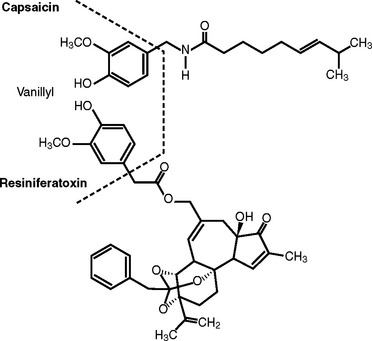
Vanilloid Pharmacology
Hot peppers have been eaten and used by humans since prehistorical times.9 Hogyes, in 1878, reported that the pungent and irritant action of capsicol, an extract of Capsicum, is mediated by sensory nerves.10 Capsaicin and its ultrapotent analogue, RTX, collectively belong to class of neurotoxic agents referred to as vanilloids. RTX is isolated from some species of Euphorbia, a cactus-like plant. These compounds are characterized by a terminal homovanilloid moiety that interacts with a specific membrane receptor (Fig. 21-1) The action of vanilloid compounds on sensory neurons is mediated via interaction of the homovanilloid moiety with the vanilloid receptor TRPV1 (transient receptor potential V1 or VR1 receptor), a nonspecific, heat-gated cation channel that mediates the influx of calcium and sodium, resulting in depolarization of nociceptive afferents to initiate a nerve impulse passing through the dorsal root ganglion into the central nervous system.11,12 Vanilloid receptors are expressed not only by small unmyelinated C fibers but also by uroepithelial cells themselves.
As intracellular calcium levels rise, voltage-sensitive calcium channels are first activated leading initially to local transmitter release, and then inhibited, serving to block the very same response. Noxious temperatures are also sensed via this mechanism, explaining the characteristic sensation of heat that is experienced when eating chili peppers.13 Therefore, capsaicin mimics the action of physiologic/endogenous stimuli that activates the “nociceptive pathway.” At the molecular level, nociception is carried out by ion channels or receptors.
Both capsaicin and RTX cause initial excitation of sensory neurons with a subsequent lasting refractory state termed desensitization. Jancso discovered that, after a period of initial intense excitation of sensory neurons, animals treated with capsaicin become unresponsive to noxious chemical stimuli and fail to develop inflammation.14,15 A large number of studies have since confirmed this finding and established capsaicin as an useful agent for the study of sensory neuron function.16
Vanilloid Potency
The Scoville heat unit scale is commonly used commercially to compare the potency of pepper strengths. Wilbur Scoville, in 1912, calibrated the potency of peppers by extracting capsicum in alcohol and diluting it until pungency was first detected after placing a drop on his tongue.17 This technique has since been standardized using high-pressure liquid chromatography (HPLC). In fact, if all known peppers were measured using this technique, their scale of pungency would range from 1 Scoville unit, for the bell pepper, to as much as 300,000 units for the habanero pepper. Pure capsaicin has a Scoville heat unit score of 16 million, and RTX registers at 16 billion Scoville heat units, which is 1000 times the potency of capsaicin (Table 21-1).
Table 21-1 Heat Level Comparisons
| Pepper or Derivative | Scoville Value |
|---|---|
| Sweet Italian bell | 0-1 |
| Pepperoncini | 100-500 |
| Jalapeno | 1000 |
| Cayenne | 30,000-50,000 |
| Thai | 50,000-100,000 |
| Jamaican hot | 100,000-200,000 |
| Habanero | 100,000-300,000 |
| Pure capsaicin | 16,000,000 |
| Resiniferatoxin (RTX) | 16,000,000,000 |
Sensitization (Acute Excitatory Effects)
On first contact with capsaicin, afferent neurons are invariably stimulated, and there seems to be no apparent difference whether the drug is applied to the peripheral or central endings or to the cell bodies of sensory neurons. Administration of vanilloids to peripheral nerve endings results in depolarization and discharge of action potentials, which in turn evokes a characteristic burning sensation via stimulation of C-fiber polymodal nociceptors. Acute activation of the sensory neurons via the vanilloid receptor TRPV1 results initially in depolarization and transmitter (peptide) release with eventual neuronal degeneration.18,19
Desensitization (Secondary Neurotoxic Effects)
After vanilloid-induced stimulation of primary afferent neurons, excitation subsides and the neurons become unresponsive to further applications of drug. Capsaicin desensitization is characterized by long-lasting, reversible suppression of sensory neuron activity.20 The rate and duration of desensitization is related to the dose and time of exposure to capsaicin and the time interval between consecutive dosings.7,21
Although C-fiber neurons have well-described afferent functions, they probably have important efferent functions as well, including local release in the periphery of substance P, neurokinin A, calcitonin gene–related peptides (CGRP), and other neuropeptides that directly and indirectly produce tissue inflammation.22–25 Desensitization of capsaicin-sensitive nerve fibers is associated with eventual depletion of transmitter neuropeptides.26,27
Clinical Results of Intravesical Capsaicin
Hypersensitivity Disorders
Maggi and coworkers reported the clinical urologic application of intravesical capsaicin.28 Intravesical instillation of capsaicin (0.1 to 10 μmol/L) in six patients with bladder hypersensitivity produced a concentration-related reduction of the first desire to void, bladder capacity, and pressure threshold for micturition. All patients reported disappearance or marked attenuation of their symptoms for a few days after capsaicin application. In three other patients, intravesical instillation of the vehicle (0.1% ethanol in saline) alone did not produce significant cystometric changes nor modify the symptomatology, suggesting that capsaicin-sensitive nerves exist in the human bladder. A second series using intravesical capsaicin, a randomized, placebo-controlled trial in patients with bladder hypersensitivity, confirmed the beneficial effect of intravesical instillation of capsaicin on voiding parameters but did not confirm improvement in pain score after capsaicin treatment compared with placebo.29
Although intravesical capsaicin has been proposed as a treatment option for interstitial cystitis, its utility has not been widely explored. One small pilot study of intravesical capsaicin in five patients with interstitial cystitis, using criteria of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), demonstrated subjective improvement in both symptom and pain score in four of them.30
Neurogenic Detrusor Overactivity
Fowler and associates reported the first clinical experience with capsaicin in neurologically impaired patients with intractable incontinence due to multiple sclerosis and spinal cord injury.31,32 After a single intravesical instillation of capsaicin (1 to 2 mmol/L) for 30 minutes, 10 of 14 patients exhibited an improved bladder capacity for up to 9 months without toxicity. Similar findings were noted in small studies in other patients with neurogenic DO.33,34
De Ridder and associates described the long-term outcome of intravesical capsaicin instillation in 79 patients with spinal cord disease and treatment-resistant urinary incontinence.35 Repeated intravesical instillation of intravesical capsaicin (1 to 2 mmol/L in 30% ethanol) was effective, and the benefit persisted for up to 3 to 5 years. In patients with phasic detrusor hyperreflexia, complete continence was achieved in 44%, satisfactory improvement occurred in 36%, and treatment failure was observed in 20%. Clinical benefit from a single instillation lasted 3 to 6 months and was repeated in some patients with similar improvement. There was no clinical or urodynamic improvement in patients treated with the ethanol vehicle alone, and there were no reported long-term complications.
There have been only a few reported randomized, placebo-controlled studies of capsaicin in patients with neurogenic DO. The results of these trials have been mixed. Wiart and colleagues reported that a single intravesical instillation of 1 mmol/L capsaicin resulted in clinical improvement with significant regression of urine leakage episodes and urgency compared with those receiving vehicle (30% ethanol) alone.36 These findings were supported by a later study in a similar population.37 A third placebo-controlled crossover study of 12 patients by Petersen and associates showed no benefit for intravesical capsaicin treatment therapy in patients with neurogenic DO.38
Resiniferatoxin
RTX is an ultrapotent capsaicin analogue present in the latex of a cactus-like plant, Euphorbia resinifera.19,39 It mimics most biologic characteristics of capsaicin with approximately 1000-fold higher potency and minimal initial acute excitatory effects.9 There are significant differences in biologic response between RTX and capsaicin. RTX and capsaicin show striking differences in relative potencies to excite and desensitize primary sensory neurons. In most cases, when RTX and capsaicin differ in potency of a particular biologic end point, the response is such that RTX preferentially causes desensitization whereas capsaicin administration leads to profound excitation.19
Intravesical RTX in concentrations as low as 100 nmol/L induced full desensitization, whereas a capsaicin concentration of 1 mmol/L was required to induce the same effect.40–42 In addition, 100 nmol/L RTX solutions were much less irritating to bladder afferents than 1 mmol/L capsaicin solutions. RTX-induced desensitization may occur at concentrations so low that no noxious effect is elicited. Because of its potency and unique property of preferential desensitization, there has been much interest in the application of RTX therapy for patients with interstitial cystitis and DO.
Clinical Results of Intravesical Resiniferatoxin
Interstitial Cystitis
The positive findings of a number of small pilot studies evaluating the efficacy of capsaicin in interstitial cystitis logically led to interest in the use RTX in the treatment of interstitial cystitis, a poorly understood condition. Most of these studies were poorly controlled, but one small, placebo-controlled study did suggest that RTX was effective in the treatment of urinary frequency, urgency, and pelvic pain.43 A recent, relatively large randomized, double-blind, placebo-controlled trial of a single dose of 0.01 to 0.10 μmol/L RTX found that it was not effective in improving overall symptoms, pain, urgency, frequency, nocturia, or voided volume during 12 weeks of follow-up.44
Neurogenic Detrusor Overactivity
The first clinical use of RTX in patients with neurogenic DO was reported by Cruz and colleagues in 1997.40 They treated seven patients with intravesical instillation of 50 to 100 nmol/L RTX dissolved in 100 mL solution of 10% alcohol. Itching or mild discomfort were the only symptoms evoked in four patients during the first minutes of the treatment. Temporary exacerbation of bladder symptoms, as seen during the first 1 to 2 weeks after capsaicin administration, did not occur. In five of the seven patients, urinary frequency decreased by 33% to 58%, and this effect was detected as soon as the first day after treatment. Three patients were incontinent and became dry on most days. Improvement was sustained for up to 3 months, the longest follow-up period available. Four patients had urodynamic improvement, with a rise in maximum cystometric capacity from 50% to 900% of pretreatment cystometric capacity.
Lazzeri and colleagues reported using intravesical RTX (10 nmol/L) in eight normal patients and seven patients with overactive bladder.42 RTX did not decrease the volume required to elicit the first desire to void and did not produce warm or burning sensations at the suprapubic or urethral level during infusion in normal subjects. Mean capacity increased significantly in patients with overactive bladder immediately after instillation but was not significantly increased after 4 weeks. There was no significant improvement in bladder capacity in the overall group, but two patients with detrusor hyperreflexia did improve urodynamically in conjunction with clinical improvement in frequency, nocturia, and incontinence episodes.
Although the results of several small studies suggested that intravesical RTX may have a role in the treatment of refractory neurogenic detrusor overactivity, its efficacy has not been conclusively confirmed in any randomized controlled trials.45–48 RTX has been demonstrated to adsorb to polyethylene, polyvinylchloride, and latex (but not silicone or glass) catheters and containers. These materials were used in some of these studies, possibly leading to lower than expected drug delivery, thereby confounding the results obtained.49
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree