Chapter 4 PHARMACOLOGIC BASIS OF BLADDER AND URETHRAL FUNCTION AND DYSFUNCTION
The main functions of the bladder and urethra are to collect and store urine at low intravesical pressure and to expel the urine at convenient times. This means that normal voiding is under voluntary control. Urinary continence and voiding depend on the bladder and the urethra working as a functional unit. This is achieved by a complex interplay between the central and peripheral nervous systems and local regulatory factors.1 Malfunction at various levels may result in micturition disorders, which roughly can be classified as disturbances of storage or emptying. Failure to store urine may lead to various forms of incontinence; the most common forms are urgency and stress incontinence. There are gender differences in these types of bladder dysfunction, which may be related to differences in anatomy and structure of the lower urinary tract.
Because pharmacologic treatment of urinary incontinence (i.e., urgency and stress incontinence) and lower urinary tract symptoms is a main option, several drugs with different modes and sites of action have been tried.2–5 To optimize treatment, knowledge about the mechanisms of micturition and of the targets for treatment is necessary. This chapter reviews the normal autonomic control of the lower urinary tract and of the pharmacologic basis for some of the principles used for treatment of urinary incontinence and lower urinary tract symptoms.
AUTONOMIC RECEPTOR FUNCTIONS IN THE BLADDER
Contraction and relaxation of detrusor and urethral smooth muscle are mediated mainly through stimulation of autonomic receptors for the main transmitters, acetylcholine and noradrenaline. However, the occurrence of nonadrenergic, noncholinergic (NANC) neurotransmission is well recognized, although, particularly in humans, its physiologic importance remains to be established.6
Muscarinic Receptors
Muscarinic receptors comprise five subtypes, encoded by five distinct genes.7 The five gene products correspond to pharmacologically defined receptors, and M1 through M5 are used to describe the molecular and pharmacologic subtypes. In the human bladder, the mRNAs for all muscarinic receptor subtypes have been demonstrated,8 with a predominance of mRNAs encoding M2 and M3 receptors.8,9 These receptors are functionally coupled to G proteins, but the signal transduction systems vary.10–12
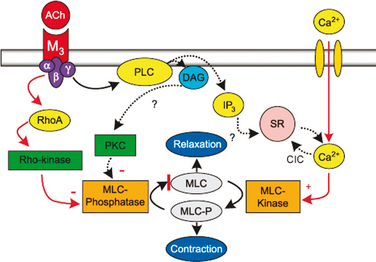
Detrusor smooth muscle contains muscarinic receptors of mainly the M2 and M3 subtypes, with a predominance (70% to 80%) of M2 receptors.10–13 The M3 receptors are the most important for detrusor contraction. It is generally believed that M3 receptors are coupled to release of inositol 1,4,5-triphosphate (IP3) and calcium release from the sarcoplasmic reticulum and that M2 receptors are linked to inhibition of adenylyl cyclase. This may not be the case in the detrusor.14 Jezior and colleagues15 suggested that muscarinic receptor activation of detrusor muscle includes nonselective cation channels and activation of Rho kinase. Supporting a role of Rho kinase in the regulation of rat detrusor contraction and tone, Wibberley and coworkers16 found that Rho kinase inhibitors (e.g., Y-27632, HA 1077) inhibited contractions evoked by carbachol without affecting the contraction response to KCl. They also demonstrated high levels of Rho kinase isoforms (I and II) in the bladder. Schneider and associates13 concluded that carbachol-induced contraction of human urinary bladder is mediated by M3 receptors and largely depends on Ca2+ entry through nifedipine-sensitive channels and activation of the Rho kinase pathway. The main pathway for muscarinic receptor activation of the detrusor by means of M3 receptors may be calcium influx through L-type calcium channels and increased sensitivity to calcium of the contractile machinery produced by inhibition of myosin light chain phosphatase through activation of Rho kinase (Fig. 4-1).14
The functional role for the M2 receptors has not been clarified, but it has been suggested that M2 receptors may oppose sympathetically mediated smooth muscle relaxation, mediated by β- adrenergic receptors.17 M2-receptor stimulation may also activate nonspecific cation channels18 and inhibit KATP channels through activation of protein kinase C.19,20 In certain disease states, M2 receptors may contribute to contraction of the bladder. In the denervated rat bladder, M2 receptors or a combination of M2 and M3 mediated contractile responses, and the two receptor types seemed to act in a facilitatory manner to mediate contraction.21–23 In obstructed, hypertrophied rat bladders, there was an increase in total and M2 receptor density, whereas there was a reduction in M3 receptor density.24 The functional significance of this change for voiding function has not been established. Pontari and colleagues25 analyzed bladder muscle specimens from patients with neurogenic bladder dysfunction to determine whether the muscarinic receptor subtype mediating contraction shifts from M3 to the M2 receptor subtype, as found in the denervated, hypertrophied rat bladder. They concluded that whereas normal detrusor contractions are mediated by the M3 receptor subtype, in patients with neurogenic bladder dysfunction, contractions also can be mediated by the M2 receptors.
Muscarinic receptors may also be located on the presynaptic nerve terminals and participate in the regulation of transmitter release. The inhibitory prejunctional muscarinic receptors have been classified as M4 in the human bladder.26 Prejunctional facilitatory muscarinic receptors appear to be the M1 subtype.27 The muscarinic facilitatory mechanism seems to be upregulated in overactive bladders from chronic spinal cord transected rats. Facilitation in these preparations is primarily mediated by M3 receptors.27,28
Muscarinic receptors have been demonstrated on the urothelium or suburothelium, but their functional importance has not been clarified.12,29 It has been suggested that they may be involved in the release of an unknown inhibitory factor.12
It is well documented2,3 that antimuscarinic agents are effective for treatment of overactive bladder, which suggests that muscarinic receptors may be involved in its pathogenesis. Contraction of the bladder, whether voluntary or involuntary, involves stimulation of the muscarinic receptors on the detrusor by acetylcholine released from activated cholinergic nerves. However, antimuscarinic agents at clinically recommended doses have little effect on voiding contractions and may act mainly during the bladder storage phase,30 during which there is normally no parasympathetic outflow from the spinal cord.31 Supporting this, antimuscarinic agents have been shown to reduce bladder tone during storage and to increase cystometric bladder capacity. A basal release of acetylcholine from non-neuronal (urothelial) and neuronal sources has been demonstrated in isolated human detrusor muscle.32 It has been suggested that this release, which is increased by stretching the muscle and in the aging bladder, contributes to detrusor overactivity and overactive bladder by eventually increasing bladder afferent activity during storage.33 This may occur because of a direct effect on suburothelial afferents or stimulation of contraction of detrusor muscle cells, which already have an increased myogenic activity in the overactive detrusor.34 Enhanced myogenic contractions can generate an enhanced afferent signal, contributing to urge or initiation of the micturition reflex.
Alpha-Adrenoceptors
α-Adrenoceptors may have effects on different locations in the bladder: the detrusor smooth muscle, the detrusor vasculature, the afferent and efferent nerve terminal, and intramural ganglia. The importance of the α1-adrenoceptors in the human detrusor in the generation of lower urinary tract symptoms has not been established. Most investigators agree on that there is a low-level expression of these receptors.35,36 In studies of the human detrusor, Malloy and coworkers36 found that two thirds of the αadrenoceptor mRNAs expressed were α1D, there was no α1B, and one third was α1A. In the rat detrusor, the α1-adrenoceptor distribution was different: the α1A-adrenoceptor was the predominant form, one third was the α1D-adrenoceptor, and there was very little of the α1B-adrenoceptor form. This was consistent in the different parts of the detrusor.37
A change of subtype distribution may be produced by outflow obstruction. Hampel and associates37 reported that there was a change in the obstructed bladder from α1A-adrenoceptor to α1D-adrenoceptor mRNA predominance. In humans, there is an α1D-adrenoceptor predominance in the normal detrusor, which means that a change in a similar direction, as in the rat, would be of minor importance provided that the number of receptors did not increase. Studies by Nomiya and colleagues38 confirmed the low-level expression of α-adrenoceptor mRNA in normal human detrusor, and they further demonstrated that there was no upregulation of any of the adrenergic receptors with obstruction. In functional experiments, they found a small response to phenylephrine at high drug concentrations with no difference between normal and obstructed bladders. In the obstructed human bladder, there seems to be no evidence for α-adrenoceptor upregulation or change in subtype. This finding was challenged by Bouchelouche and coworkers,39 who found an increased response to α1-adrenoceptor stimulation in obstructed bladders. Whether this would mean that the α1D-adrenoceptors in the detrusor muscle are responsible for detrusor overactivity or overactive bladder is unclear. Based on available evidence, however, it does not seem likely that in the detrusor muscle these receptors should be an important treatment target, although α1D-adrenoceptors located elsewhere in the bladder may be important.
In the bladder, the function of the detrusor muscle depends on the vasculature and on perfusion. Hypoxia induced by partial outlet obstruction is believed to play a major role in the hypertrophic and degenerative effects of partial outlet obstruction. Das and associates40 investigated in rats whether doxazosin affected blood flow to the bladder and reduced the level of bladder dysfunction induced by partial outlet obstruction. They found that 4 weeks of treatment with doxazosin increased bladder blood flow in control and obstructed rats. Doxazosin treatment also reduced the severity of the detrusor response to partial outlet obstruction. Doxazosin may reduce the increase in bladder weight in obstructed animals, which may be one of the mechanisms that contributes to a positive effect on detrusor overactivity caused by the obstruction.
Beta-Adrenoceptors
In the human detrusor, the most important β-adrenoceptor for bladder relaxation is the β3-adrenoceptor.41 This partly explains why the clinical effects of selective β2-adrenoceptor agonists in detrusor overactivity have been controversial and largely inconclusive.2,3 However, the β2-adrenoceptor agonist clenbuterol inhibited electrically evoked contractions in human “unstable” but not normal bladder,42 which is in agreement with previous experiences in patients, suggesting that clenbuterol and other β2-adrenoceptor agonists such as terbutaline may inhibit detrusor overactivity.43,44
The β3-adrenoceptor seems to be an interesting target for drugs aimed for treatment of overactive bladder. Selective β3-adrenoceptor agonists have relaxant effects on detrusor muscle in vitro and have been effective in animal models of detrusor overactivity.45–47 However, no proof of concept studies seems to have been performed in humans showing that this is an effective principle to treat overactive bladder.
Purinergic Receptors
In most animal species, bladder contraction induced by stimulation of nerves consists of an atropine-sensitive component and a component mediated by NANC mechanisms.48 NANC-mediated contractions have been reported to occur in normal human detrusor,48 even if not representing more than a few percent of the total contraction in response to nerve stimulation. However, a significant degree of NANC-mediated contraction may exist in morphologically or functionally changed human bladders, and it has been reported to occur in several disorders associated with lower urinary tract symptoms, such as bladder hypertrophy,49–51 idiopathic detrusor overactivity,51,52 interstitial cystitis,53 and neurogenic damage54 and in the aging bladder.55 In these disorders, the NANC component of the nerve-induced response may be responsible for up to 40% to 50% of the total bladder contraction.
There is good evidence that the transmitter responsible for the NANC component is adenosine triphosphate (ATP)56 acting on P2X receptors found in the detrusor smooth muscle membranes of rats57 and humans.58 The P2X1receptor subtype predominated in both species. Changes in P2X receptor subtypes in bladders from patients with idiopathic detrusor overactivity have been reported.52,59 O’Reilly and colleagues52 were unable to detect a purinergic component of nerve-mediated contractions in control (normal) bladder preparations, but they found a significant component in overactive bladder specimens, in which the purinergic component was approximately 50%. They concluded that this abnormal purinergic transmission in the bladder might explain symptoms in these patients. ATP was a more potent contractile agonist in bladder preparations from patients with overactive and obstructive bladders than in specimens from normal bladders,60 a finding that was suggested to contribute to detrusor overactivity.
O’Reilly and coworkers61 confirmed that the P2X1 receptor was the predominant purinoceptor subtype in the human male bladder. They also found that the amount of P2X1 receptor per smooth muscle cell was greater in the obstructed than in control bladders. This suggests an increase in purinergic function in the overactive bladder arising from bladder outlet obstruction.
Vanilloid Receptors
Appropriate bladder function depends on an intact afferent signaling from the bladder to the central nervous system. This signaling conveys information about bladder filling and the status of the tissue (e.g., presence of infectious agents). The afferent nerves consist of small, slowly conducting, myelinated Aδ fibers and slowly conducting, unmyelinated C fibers. The former are excited by mechanoreceptors and convey information about bladder filling, whereas C fibers mediate painful sensations recognized by chemoreceptors. By means of capsaicin, a subpopulation of primary afferent neurons innervating the bladder and urethra (i.e., capsaicin-sensitive nerves) has been identified. It is believed that capsaicin exerts its effects by acting on specific vanilloid receptors on these nerves.62 Capsaicin exerts a biphasic effect; initial excitation is followed by a long-lasting blockade, which renders sensitive primary afferents (C fibers) resistant to activation by natural stimuli. In sufficiently high concentrations, capsaicin is believed to cause desensitization, initially by releasing and emptying the stores of neuropeptides and then by blocking further release.63 Resiniferatoxin, an analogue of capsaicin, is approximately 1000 times more potent for desensitization than capsaicin,64 but it is only a few hundred times more potent for excitation.65 Capsaicin and resiniferatoxin also may have effects on Aδ fibers. It is also possible that capsaicin at high concentrations (mM) has additional, nonspecific effects.66
Capsaicin and resiniferatoxin have been used successfully to treat bladder function disturbances.2,3 They are known to bind to the TRPV1 (VR1) receptor, a nonselective cation channel, on the peripheral terminals of nociceptive neurons, but the role of vanilloid receptors in normal bladder function and in the pathogenesis in detrusor overactivity and autonomic dysreflexia has not been established.
Birder and associates67 investigated bladder function in conscious mice lacking the TRPV1 receptor. TRPV1 receptor–knockout mice seem to have increased voiding frequency compared with wild-type mice. The TRPV1 knockouts also had an increased frequency of nonvoiding bladder contractions. In vitro, stretch-induced ATP release was decreased in bladders from TRPV1-knockout mice, and hypo-osmolality-induced ATP released from cultured urothelial cells was reduced.67 The investigators suggested that TRPV1 receptors participate in normal bladder function, are essential for normal mechanically evoked purinergic signaling by the urothelium, and are involved in ATP release. Experimental and clinical evidence that capsaicin-sensitive afferents may be involved also in idiopathic detrusor overactivity has been presented.68,69
ION CHANNELS
In the detrusor, the two most thoroughly investigated classes of ion channels are calcium channels and potassium channels.6
Calcium Channels
Calcium is a key component for cell function in many cells. In smooth muscle, increased intracellular calcium concentrations activate the contractile mechanisms, and in nerve terminals, calcium influx in response to action potentials is an important mechanism for neurotransmitter release. Calcium channels can be divided into at least four different subtypes: L, N, P, and Q channels. The calcium channels present in smooth muscles are L-type (dihydropyridine-sensitive) calcium channels and seem to be involved in contraction of the human bladder irrespective of the mode of activation.70 A decrease of the membrane potential (i.e., depolarization) increases the open probability for calcium channels, thereby increasing the calcium influx. The channels depend on the membrane potential and are called voltageoperated calcium channels. Elevated intracellular calcium levels are also believed to initiate release of calcium from intracellular stores—calcium-induced calcium release.71,72 Regulation of the intracellular calcium concentration in smooth muscle cells is a conceivable way to modulate bladder contraction. Dihydropyridines (e.g., nifedipine) have a potent inhibitory effect on isolated detrusor muscle. Inhibitory effects have also been demonstrated on experimentally induced contractions under in vivo conditions in rats and clinically in patients with detrusor overactivity.48 However, therapeutically, there is no evidence that calcium antagonists have any useful effects in the treatment of overactive bladder or detrusor overactivity.2,3
Potassium Channels
Potassium channels represent another mechanism for modulating the excitability of smooth muscle cells. Under normal conditions, the resting membrane potential in smooth muscle cells is determined predominantly by the membrane conductivity for potassium ions. Increased potassium conductivity lowers the membrane potential by increasing the potassium efflux. This increases the threshold for opening of voltage-operated calcium channels and initiation of contraction. There are several different types of K+ channels, and at least two subtypes have been found in the human detrusor, ATP-sensitive K+ channels (KATP) and large-conductance calcium-activated K+ channels (BKCa). Studies on isolated human detrusor muscle and on bladder tissue from several animal species have demonstrated that K+-channel openers reduce spontaneous contractions and contractions induced by carbachol and electrical stimulation.6 However, the lack of selectivity of the available K+-channel openers for the bladder versus the vasculature has limited the use of these drugs. No effects of cromakalim or pinacidil on the bladder were found in studies on patients with spinal cord lesions or detrusor overactivity secondary to outflow obstruction.73,74 Some new K+-channel openers have been developed and claimed to have selectivity for the bladder.6 However, there is no evidence that K+-channels openers are an option for treatment of overactive bladder or detrusor overactivity.2,3
AFFERENT SIGNALING FROM THE UROTHELIUM OR SUBUROTHELIUM
Evidence suggests that the urothelium or suburothelium may serve as a mechanosensor that, by producing nitric oxide, ATP, and other mediators, can control the activity in afferent nerves and thereby the initiation of the micturition reflex.75 Low pH, high K+ concentration, increased osmolality, and low temperatures can influence afferent nerves, possibly through effects on the vanilloid receptor (i.e., capsaicin-gated ion channel [TRPV1]), which is expressed in afferent nerve terminals and in the epithelial cells that line the bladder lumen.67,76 A network of interstitial cells, extensively linked by connexin43 (Cx43)–containing gap junctions, was found to be located beneath the urothelium in the human bladder by Sui and colleagues77,78 This interstitial cellular network was suggested to operate as a functional syncytium, integrating signals and responses in the bladder wall. The firing of suburothelial afferent nerves, conveying sensations and regulating the threshold for bladder activation, may be modified by inhibitory (e.g., nitric oxide) and stimulatory (e.g., ATP, tachykinins, prostanoids) mediators. ATP, generated by the urothelium, has been suggested as an important mediator of urothelial signaling.56,75 Supporting such a view, intravesical ATP induces detrusor overactivity in conscious rats.79 Mice lacking the P2X3 receptor were shown to have hypoactive bladders.80,81
There seem to be other, unidentified factors in the urothelium that may influence bladder function.82–89 Fovaeus and coworkers85 found that a previously unrecognized nonadrenergic, nonnitrergic, nonprostanoid inhibitory mediator is released from the rat urinary bladder by muscarinic receptor stimulation. However, it was not clear whether this factor came from the detrusor muscle or from the detrusor and the urothelium. Hawthorn and associates86 presented data suggesting the presence of a diffusible, urothelium-derived inhibitory factor, which could not be identified but appeared to be neither nitric oxide, a cyclooxygenase product, a catecholamine, adenosine, γ-aminobutyric acid (GABA), nor any substance sensitive to apamin. The identity and possible physiologic role of the unknown factors remain to be established and should offer an interesting field for further research. These mechanisms can be involved in the pathophysiology of overactive bladder and may be useful targets for pharmacologic intervention.
URETHRAL FUNCTION AND STRESS URINARY INCONTINENCE
Many factors are involved in the pathogenesis of stress urinary incontinence (SUI). Some, such as weak urethral support, vaginal prolapse, and severe vesical neck or urethral dysfunction,90 cannot be treated pharmacologically. Women with SUI have lower resting urethral pressures than age-matched, continent women,91,92 and the aim of treatment often is to increase intraurethral pressure.
Factors that may contribute to urethral closure include urethral smooth and striated muscle tone and the passive properties of the urethral lamina propria, in particular the vascular suburothelial layer. The relative contribution to intraurethral pressure of these factors remains a subject of debate. In one study, the contributions to the total intraurethral pressure of the striated muscle component in the urethra and pelvic floor, the urethral vascular bed, and the smooth musculature and connective tissues in urethra and periurethral tissues were found to be one third each.93
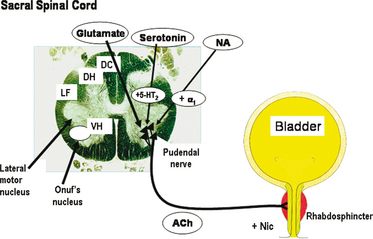
Many factors have been suggested to contribute to urethral relaxation and to urethral closure, including urethral smooth muscle tone and the properties of the urethral lamina propria. There is ample pharmacologic evidence that a substantial part of urethral tone is mediated through stimulation of αadrenoceptors in the urethral smooth muscle by released noradrenaline.48 Nitric oxide has emerged as an important mediator of urethral smooth muscle relaxation, but the roles of other transmitters cannot be excluded.94–96 However, central nervous control of the smooth and striated urethral muscle is important for the maintenance of continence.97–99 The nucleus Onuf is the spinal target of reuptake inhibitors of serotonin (5-hydroxytryptamine) and noradrenaline, which may increase the tone of the striated sphincter (Fig. 4-2).