Technique
Methods
Aspiration
Contrast, alcohol
Biopsy
Cytology, core biopsies
Catheter guidance
Drainage: cholecystostomy, abscess, etc.
Definition of intra-abdominal anatomy
Fluid collections (e.g., blood, puss, cyst, etc.)
Injection
Operative approach
Probe guidance
Tumor ablation
Interventional procedures guided by ultrasound for needle placement include biopsy, aspiration, and injection/ablation. Both fine-needle aspiration (FNA) cytology and core-needle biopsy can be guided by ultrasound. Ultrasound-guided FNA cytology is usually indicated for neck masses, including thyroid nodules, parathyroid nodules, cervical or other lymph nodes, and pancreatic lesions. On the other hand, ultrasound-guided core-needle biopsy is indicated more commonly for breast lesions, abdominal diseases such as liver tumors, retroperitoneal or pelvic masses, prostate lesions, and transplanted organs [12–14]. For both FNA and core-needle biopsy, ultrasound greatly facilitates the diagnosis and management of hepatic disorders, particularly neoplasms. Ultrasound-guided biopsy of the liver can be performed percutaneously in most circumstances and is frequently indicated for definitive diagnosis of liver tumors [15] (Fig. 7.1). Percutaneous biopsy of intra-abdominal, retroperitoneal, or pelvic masses can be guided by ultrasound as well as computed tomography (CT). Percutaneous ultrasound-guided biopsy of tumors of the liver or intra-abdominal masses may confirm metastatic or unresectable disease, thereby avoiding unnecessary laparotomy in a patient with incurable malignancy [15]. Ultrasound can be used to biopsy retroperitoneal masses including lymph nodes [16], even preventing unnecessary laparotomy in a patient with retroperitoneal lymphoma (Fig. 7.2). Ultrasound-guided core-needle biopsy of transplanted organs such as the liver, kidney, and pancreas is indicated for histologic diagnosis of possible transplant rejection or other transplantation-related complications [17].
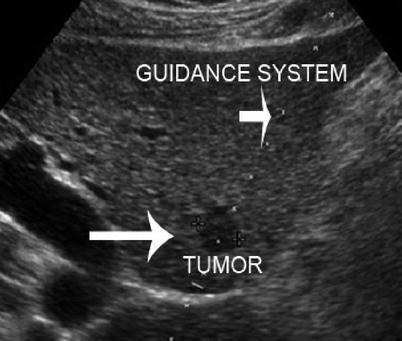
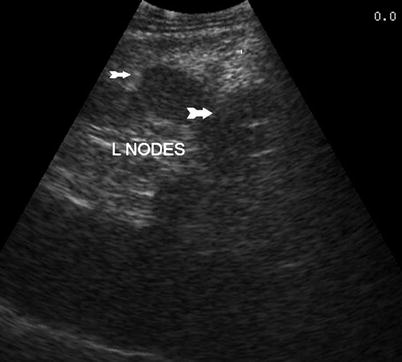

Fig. 7.1
Biopsy of liver tumor

Fig. 7.2
Retroperitoneal lymph nodes (arrows)
Ultrasound-guided needle placement is also indicated for aspiration of various fluids and cystic lesions involving the thoracic and abdominal cavities as well as other areas of the body. Ultrasound-guided aspiration is performed for both diagnostic and therapeutic purposes in the abdomen, for example, a paracentesis, and is much safer for the patient when done under ultrasound guidance. Abdominal cystic lesions that can be aspirated by ultrasound-guided needle placement are numerous and include, but are not limited to, liver cysts, renal cysts, and pancreatic cystic lesions [18] (Fig. 7.3).
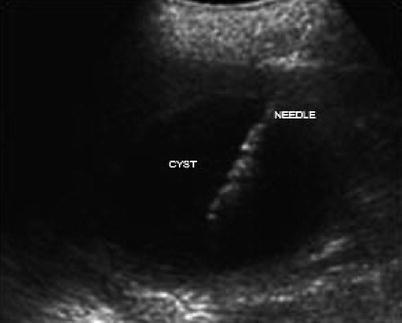

Fig. 7.3
Ultrasound-guided cyst aspiration
Ultrasound-guided needle aspiration may be followed by ultrasound-guided catheter placement for drainage of various diseases. Intra-abdominal or pelvic abscesses and cysts can be aspirated and drained (Figs. 7.4 and 7.5). Some interventions require the placement of a catheter including percutaneous cholecystostomy, as well as percutaneous transhepatic cholangiography (PTC), nephrostomy, gastrostomy, and aspiration and drainage of abdominal wall and intra-abdominal fluid collections (Fig. 7.6).
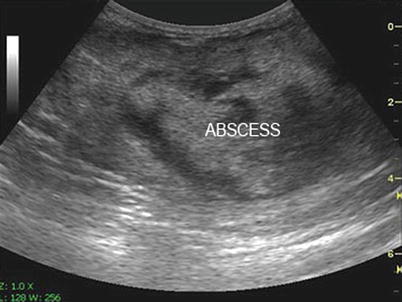
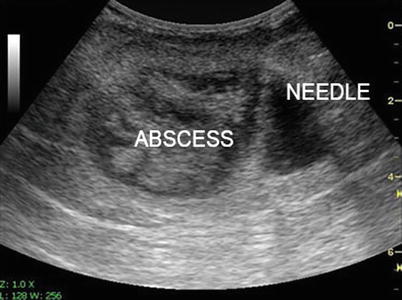
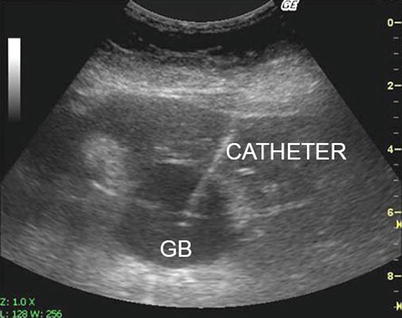

Fig. 7.4
Intra-abdominal abscess detected with ultrasound

Fig. 7.5
Ultrasound-guided needle placement for abscess drainage

Fig. 7.6
Percutaneous cholecystostomy. GB Gallbladder
Injection and/or tissue ablation of lesions is an important concept when discussing interventional sonography. Following needle placement, various agents can be injected under ultrasound guidance. Such agents include blue dye, radiographic contrast, or therapeutic agents such as alcohol. Blue dye injection may be used for marking the tissue or in the case of hepatic resections, injection into a branch of the portal vein to define the boundaries of a liver segment to facilitate subsegmental resection. Contrast can be injected into cystic lesions or biliary ducts for radiographic contrast studies (e.g., percutaneous transhepatic cholangiography). Ultrasound-guided ethanol injection has been used for management of hepatomas, parathyroid adenomas, thyroid nodules, and other diseases [19].
Tumor ablation is an effective therapeutic modality for the management of various types of cancers. Its success is dependent on accurate staging, precise targeting of the lesion, thorough ablation, and consecution of free margins. Cryoablation and thermal and microwave ablation have been extensively utilized in the treatment of liver tumors with good outcomes. Placement of the probes can be guided by ultrasound in an accurate, expeditious, and safe manner. Radiofrequency/microwave-ablated or cryoablated lesions become hyperechoic or hypoechoic, respectively, and associated with shadowing (Figs. 7.7 and 7.8) [20, 21].
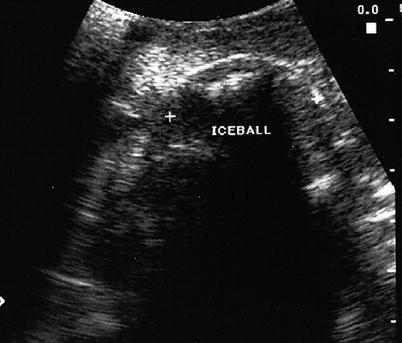
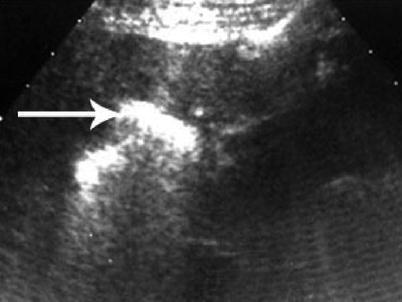

Fig. 7.7
Cryoablated liver lesion. Iceball between crossmarks

Fig. 7.8
Post-RFA of liver mass with hyperechoic residual effect
Advantages and Disadvantages
With the wide variety of imaging modalities available to the surgeon presently, it is sometimes challenging to select the most effective method. What is best for the patient? Which modality will yield the greatest amount of information to guide clinical decision-making? Which one is the most cost-effective? (Table 7.2) The advantages of ultrasonography are numerous. The most significant is its visualization of an interventional procedure in real time, which allows for precise needle or cannula placement [22]. Ultrasound is a dynamic study with real-time imaging allowing for controlled intervention with a needle, catheter, etc. under visualization. With the exception of fluoroscopy, other image guidance modalities (e.g., CT and magnetic resonance imaging) require assessment of static images and, when necessary, subsequent adjustment of needle placement prior to rescanning. Precise placement of the needle and/or probe requires accurate definition of the target. If subsequent CT or magnetic resonance imaging (MRI) images are obtained without contrast, the risk for unsuccessful outcomes due to poor delineation of the lesion may be increased. Ultrasound also has the ability to be portable, which is extremely useful in the intensive care unit (ICU) setting or any setting in which a critically ill patient is involved. This minimizes any risk of transportation in a critically ill patient, and there is less of a burden on the collaborating departments (e.g., radiology) and the nursing staff. The portability of ultrasound also makes it ideal for the office or ambulatory setting. Focused assessment with sonography for trauma (FAST) in the emergency department to diagnose bleeding in the trauma patient has become extremely common and effective (Fig. 7.9). The uses of ultrasound to perform interventions due to its accessibility and portability will only continue to grow. Finally, ultrasound is considerably less expensive than CT, MRI, and other imaging modalities.
Table 7.2
Advantages and disadvantages of interventional ultrasonography
Advantages | Disadvantages |
|---|---|
Accurate | Difficult to image mid-abdomen and chest |
Available | Interference from air and bone |
Doppler capability | Learning curve |
No ionizing radiation | Lower resolution |
Portability | Sterilization of probes |
Real-time visualization/dynamic study | |
Repeatable: confirms procedure, complications | |
Safe (no redirection) | |
Versatile |

Fig. 7.9
Portable ultrasound used in the emergency department for FAST scan
One of the main advantages to ultrasound is its lack of ionizing radiation. As a result, it can be used in circumstances in which image guidance would otherwise not be possible; examples include pregnant patients or radiation-sensitive areas (e.g., ultrasound-guided aspiration of amniotic fluid). Ultrasound also has the capability for immediate confirmatory imaging post-procedure. Successful aspiration of the fluid or drainage can be confirmed. Tumor-ablative procedures can be monitored by continuous ultrasound examination during the operation. During or at the end of the procedure, ultrasound may be used to detect early complications such as bleeding at the needle insertion site or hematoma formation [22].
Despite its myriad of uses and the abovementioned advantages, there are some disadvantages to ultrasonography [23]. Because ultrasound does not penetrate bone or air well, ribs or air within the lung, limits its use in evaluating the chest. Specifically in the abdomen, gas within the bowel lumen limits the value of ultrasound in evaluating the mid-abdominal region. Therefore, in most circumstances, CT guidance is required for intervention in those areas. The cumbersome mechanism for disinfection and sterilization of ultrasound transducers is a minor impediment to their use. Current transducers have limited tolerance to high temperature, preventing their sterilization by autoclaving. Alternative techniques necessitate time-consuming cold gas sterilization or soaking procedures with or without the use of sterile drape covers [20].
Procedure Preparation
As with any interventional procedure, routine background information with attention to a history of bleeding disorders, liver disease, and medications is required. Specifically, the use of platelet-inhibiting drugs (e.g., aspirin, clopidogrel, etc.) and anticoagulants (e.g., warfarin, etc.) should be investigated. A review of the patient’s coagulation profile including the prothrombin time (PT) and partial thromboplastin time (PTT) are always recommended before an interventional or invasive procedure. Informed consent must always be obtained before a procedure, outlining the procedure itself as well as the potential complications and morbidities associated with the specific procedure.
In most cases, optimal patient position for interventional ultrasound procedures is the same as that used for diagnostic ultrasonography of the area. In general, one should attempt to take the shortest possible path to a lesion. For example, to optimally visualize lesions in the abdomen (such as the liver), it may be necessary to move the patient from the supine to the left lateral decubitus position [22]. Raising the patient’s right arm over the head, thereby moving the rib cage in a more cephalad direction, may further enhance exposure of the liver. Such maneuvers may be required to optimally visualize and access a liver mass. For most interventional ultrasound procedures, it is beneficial to perform a brief diagnostic scan of the patient prior to setting up for the planned intervention. This preliminary scan confirms the safest and most direct route to the area in question. When such a position is ascertained, the patient can be prepared in the usual manner (Fig. 7.10).
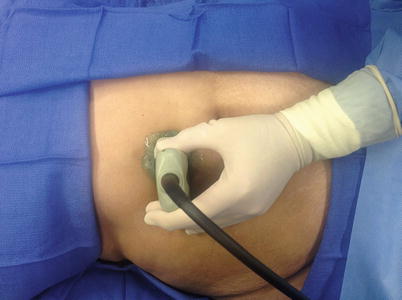

Fig. 7.10
Preliminary scan of abdomen prior to needle aspiration
Skin preparation for ultrasound-guided procedures requires routine painting with antiseptic solutions and full operative drape of the patient and ultrasound equipment. The method will vary according to the nature and severity of the planned interventional ultrasound procedure. For acoustic coupling between the transducer and the skin, sterile ultrasound coupling gel can be used. During open surgical procedures, saline solution is used in the operative field for acoustic coupling.
The use and type of anesthesia varies depending on the nature and extent of the procedure and the anxiety and cooperation of the patient. Most percutaneous interventional ultrasound procedures are accomplished readily with a of local anesthetic. For more complex procedures, such as drainage of an intra-abdominal abscess or percutaneous biliary drainage, or for patients with anxiety, intravenous sedation is generally required (typically with a narcotic and benzodiazepine). When using sedation, the patient must be in a monitored setting, watching heart rate, blood pressure, respiratory rate, and oxygen saturation. Prolonged ultrasound-guided procedures, such as liver tumor ablation, are more frequently performed under general anesthesia.
Ultrasound Guidance and Visualization
Ultrasound guidance was a major advancement in the area of interventional sonography. Saitoh et al. first described the use of real-time guidance for sonographically guided puncture in 1979 [24]. Shortly thereafter, almost every manufacturer developed some type of transducer or needle guidance system for biopsy or aspiration techniques. There are advantages and disadvantages to each guidance system. Presently, the use of detachable guidance systems for sonographically guided needle puncture techniques is common, including the development of endoluminal transducers with biopsy guidance attachments to use with these transducers. These guidance techniques have become increasingly specialized and the equipment very specific for various ultrasound-guided procedures.
Various ultrasound guidance methods are utilized to optimally guide a needle, cannula, or probe. Indirect ultrasound guidance uses the ultrasound to select the site of intervention and aids in determining the angle of insertion for the needle. Despite this, it is essentially a blind technique. First, the size and the exact location of the lesion are evaluated by ultrasound. The needle puncture site is selected and marked with a marking pen or other convenient tool. The direction and depth of the needle insertion are determined by ultrasound. The needle is inserted from the predetermined site without concomitant use of ultrasound visualization. This method is less precise than direct ultrasound guidance, and thus inferior. However, skin and equipment preparation is easier because disinfection or sterilization of the ultrasound transducer is not needed. An indirect ultrasound guidance method is generally used when the target lesion is relatively large, e.g., aspiration or drainage of large fluid collections such as ascites, a large cystic lesion, or biopsy of a large tumor (Figs. 7.11 and 7.12).
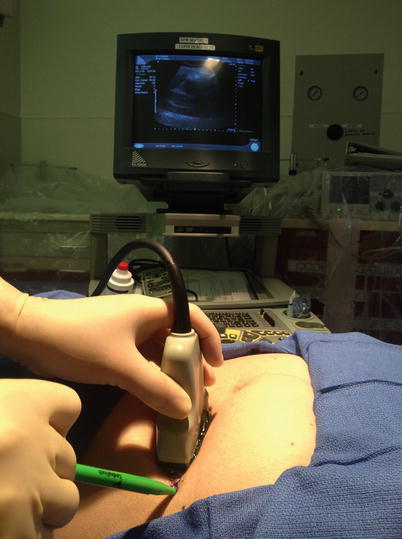
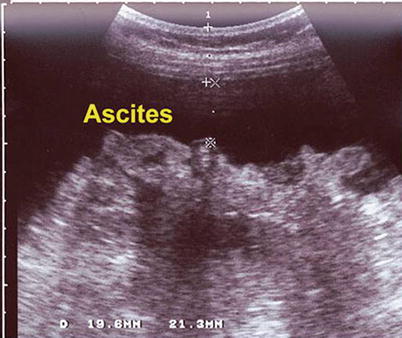

Fig. 7.11
Indirect ultrasound guidance method showing ascites

Fig. 7.12
Indirect ultrasound guidance method showing ascites
Direct ultrasound guidance methods allow real-time needle visualization and therefore more precise needle placement. There are two techniques of needle insertion in relation to the ultrasound scanning plane along the long axis of the transducer. The better and preferred technique is to insert and advance a needle in the plane parallel to the long axis of the transducer (i.e., ultrasound scanning plane). This allows for constant visualization of the shaft and tip of the needle throughout its entire path to the target. This is a safer and more accurate method because the needle tip is always demonstrated on the ultrasound monitor. The second and less preferable technique is to insert the needle perpendicular to the long axis of the transducer. This technique increases the difficulty of needle tip visualization because the tip is not seen until it enters the ultrasound scanning plane (Fig. 7.13a, b). This may increase the risk of significant past pointing of the needle tip, which may lead to complications such as pneumothorax and bleeding. For this reason, the use of this second technique should be limited to the situation in which the placement of transducer or access of the needle to the target cannot be achieved by the first technique [22].
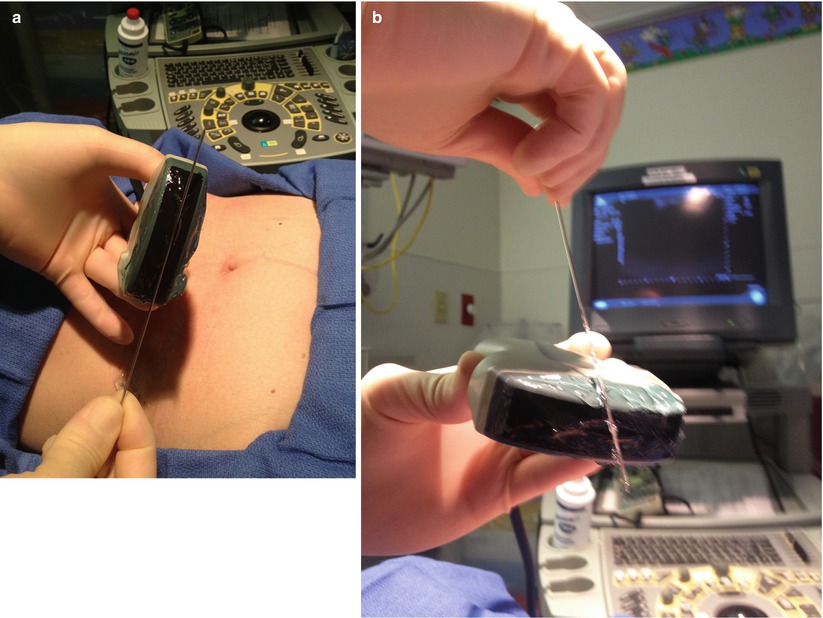

Fig. 7.13
(a, b) Two techniques of needle insertion: (a) demonstrates parallel insertion and (b) demonstrates perpendicular insertion to long axis of ultrasound scanning plane
Direct ultrasound guidance is the preferred method in interventional ultrasonography, and it can be accomplished typically in one of two ways: the “freehand” technique (Fig. 7.14) or by using the needle guidance system (so-called biopsy guide) (Fig. 7.15). In the freehand technique, the needle can be placed either adjacent to or remote from the transducer, and parallel or perpendicular to the ultrasound scanning. Another advantage of the freehand technique is that it allows independent movement of the transducer and the needle. The main disadvantage of the freehand technique is that it is sometimes difficult to keep or advance the needle within the ultrasound scanning plane for needle visualization. Various types of needle guidance systems are available. In this system, the needle is in a fixed location relative to the transducer. As a result, this guidance system allows for placement of the needle precisely along a predetermined course into the target site, which is often displayed as a needle guideline on the ultrasound monitor (Fig. 7.16). However, because of the fixation of the needle, it cannot be inserted from a remote site, which limits the usefulness of this system. Also, the transducer/needle devices can be somewhat cumbersome and more difficult to use.
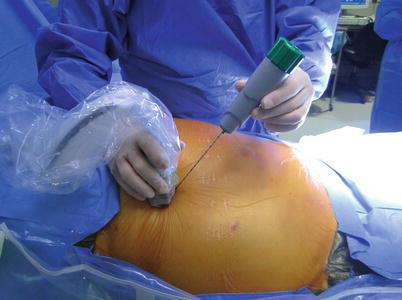

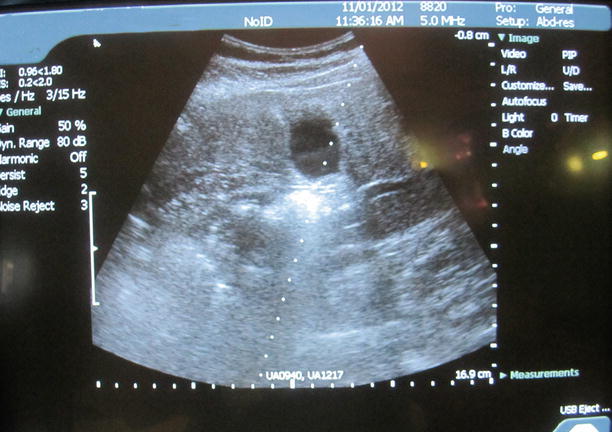

Fig. 7.14
Freehand technique

Fig. 7.15
Transducer equipped with needle guidance system

Fig. 7.16
Display of predetermined course into the target site on the monitor
Despite which technique the surgeon chooses to employ, it is the experience of the surgeon or the operator with that system that is paramount. The location and size of the target lesion will also aid one in determining which system to use. In general, more superficially located organs or lesions such as the thyroid or breast can be approached by the freehand technique. On the other hand, deeply situated lesions in locations such as the intra-abdominal organs often require a needle guidance system to access (Fig. 7.17).

Fig. 7.17
Low-frequency transducer with needle-guided system used for percutaneous cholecystostomy
Equipment
The transducers used for interventional ultrasound procedures have been either those that are biopsy dedicated or those that have the potential for needle guidance system (biopsy guide) attachment. Biopsy-dedicated transducers have fallen out of favor, primarily as a result of difficulty in needle visualization using such a transducer, but also because of the considerable difficulty in sterilizing the transducer’s central canal. Needle guidance systems are available in multiple shapes and sizes to accommodate various types of transducers. Most systems hold the needle firmly in place along a predetermined angle. Some have variable angles of insertion, which greatly facilitates appropriate and safe approach of the needle into targets. Some systems keep the needle in the ultrasound scanning plane, but the insertion angle is not fixed. Needle guidance systems either can be reusable and require cleaning/sterilization or can be disposable.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







