Chapter 85A Percutaneous methods for ablating liver tumors
Overview
Overview
The term liver tumor generally includes a variety of primary and metastatic tumors involving the hepatic parenchyma. More than 90% of the primary tumors are hepatocellular carcinomas (HCCs); the other 10% include cholangiocarcinomas and rare tumors such as hemangioendotheliomas, lymphomas, and sarcomas (McGlynn et al, 2001). Hepatic metastases (METs) can originate from all types of primary tumors, but excluding local and regional lymph nodes, the liver is usually the first organ targeted by tumors of the gastrointestinal tract (Wiess, 1986).
Background
Percutaneous Ablation of Hepatocellular Carcinoma
HCC is the fifth most common tumor throughout the world, and its incidence is increasing worldwide because of the spread of infections caused by the hepatitis B and C viruses (see Chapters 64 and 80). Surveillance programs have been developed for patients with cirrhosis, and this approach is associated with annual HCC detection rates ranging from 3% to 8% (Bruix & Llovet, 2002). Unfortunately, although advances in imaging technology have improved the early detection of HCC, these tumors are difficult to treat and are still associated with a poor prognosis.
Conventional approaches, such as systemic chemotherapy and radiation, have proved to be ineffective in HCC, although the results of some recent randomized trials suggest that sorafenib may be associated with slightly improved survival in patients with advanced HCC (Llovet et al, 2008; Peck-Radosavljevic et al, 2010; Vitale et al, 2009; see Chapter 88). Surgical resection can result in tumor eradication and improved survival in patients with small HCCs; however, few patients are candidates for potentially curative resection, which comes with an 80% risk of recurrence (Akriviadis et al, 1998; see Chapter 90B). Liver transplantation is the ideal approach for early HCC, but it is limited by age-related contraindications and a shortage of organs (Llovet et al, 1999; see Chapter 97D). For these reasons, a number of percutaneous methods have been developed for the chemical or thermal ablation of HCC, and all have displayed satisfactory efficacy in terms of local tumor control.
Percutaneous Ablation of Hepatic Metasteses
Percutaneous ablation has been used to treat hepatic METs from primary tumors in various organs, but its systematic use has been limited to those produced by colorectal cancers (CRCs). CRC is the fourth most common tumor worldwide (Greenlee et al, 2000), and the healthy liver is the first site of metastatic involvement in patients with CRC and a source of further spread (Lenhert, 1999). Twenty-five percent of these patients already have liver METs when the primary tumor is diagnosed, and another 50% develop them within 5 years of diagnosis (see Chapter 81A). In more than 30% of the patients with liver METs, the metastatic disease appears to be confined to the liver at the time of detection.
Throughout the world, resection is considered a potentially curative approach for the treatment of CRC liver METs, and it is associated with a 5-year survival rate of 40% (Ballantyne, 1993; Greenway, 1988; Nakamura, 1997). However, more than 70% of patients are ineligible for surgical resection because of the extent of their disease, serious comorbidities, or both. For those who do undergo resection, recurrence is frequent; repeat resections are difficult to propose, and in some cases, they are ineffective. Chemical or thermal ablation methods have been proposed for patients with no surgical prospects, for those with recurrence after resection or tumor progression after systemic or local chemotherapy, and for those who refuse surgery. Chemical ablation has displayed limited efficacy in the local control of liver METs (Livraghi et al, 1991), but thermal ablation has proved to be safe and effective for the treatment of small liver METs from CRC (Lencioni et al, 2004; Livraghi et al, 2003a; Solbiati et al, 1999). It has also produced promising results in the management of lung METs (Rossi et al, 2006). Furthermore, thermal ablation coupled with open surgery seems to increase the overall number of treatable patients (Abdalla et al, 2004).
Pretreatment Studies and Patient Selection
For HCC patients, treatment eligibility screening generally involves routine laboratory tests, abdominal ultrasonography (US), contrast-enhanced US, spiral computed tomography (CT) or magnetic resonance imaging (MRI), esophagogastroduodenoscopy, and other studies as indicated. Diagnosis of cirrhosis is generally based on histology or concordant laboratory and imaging findings. Most investigators believe that HCCs more than 2 cm in diameter can be reliably diagnosed on the basis of characteristic findings documented with at least two imaging modalities. One modality is sufficient if the patient also has an α-fetoprotein (AFP) level greater than 200 ng/mL. For HCC nodules less than 2 cm in diameter, diagnosis requires typical findings of two imaging techniques and AFP levels above 200 ng/mL. Otherwise, biopsy is mandatory (Bruix & Llovet, 2002). We and others believe that diagnosis of the original tumor should be based on histologic examination of US-guided fine needle biopsy (FNB) or laparoscopic findings (subcapsular tumors), although imaging findings can be useful for recurrences (Rossi et al, 1996, 1998). US-guided biopsy, cutting or aspirative, with an 18- to 21-gauge needle is always indicated when the AFP level is above 200 ng/mL.
Laboratory screening for CRC liver METs is similar to that described above, and diagnosis is generally based on imaging findings. In a patient with a history of CRC, a focal hepatic lesion is generally regarded as metastatic if it displays peripheral contrast enhancement in the arterial phase and complete washout in the portal and late phases on at least two types of imaging studies. US and FNB confirmation should be obtained if the diagnosis is in doubt, and it can also be used to identify the treatment-relevant receptor status of tumor cells (Midorikawa et al, 2009). Patients with CRC liver METs should always undergo colonoscopy before thermal ablation to exclude the possibility of local disease recurrence.
Candidates for percutaneous ablation have tumors with characteristics similar to those of patients who are eligible for surgical resection, but for a variety of reasons, they are not considered suitable for surgery. Thermal/chemical ablation is generally chosen to treat HCC on the basis of findings related to the tumor, residual liver function, and the patient’s general health. The criteria most commonly used include documented cirrhosis; no more than three HCC nodules, measuring no more than 3.0 to 3.5 cm each in diameter; no neoplastic thrombosis of the portal or hepatic veins; and no evidence of extrahepatic metastases. Residual liver function is classified according to the Child-Turcotte-Pugh (CTP) system, and patients with scores higher than B7 to B9 are usually considered unsuitable for ablation. Candidates must also have safe coagulation parameters—prothrombin time (PT) ratio of 50% or higher, international normalized ratio (INR) no higher than 1.7, and a platelet count of 50 to 70 x 109/L or more—and no esophageal varices at a high risk for bleeding (Ebara et al, 1995; Rossi et al, 1998).
Similar criteria are used to select patients with CRC liver METs for percutaneous thermal ablation (Rossi et al, 1996; Solbiati et al, 1999). The patient’s coagulation status still needs to be assessed, because liver disease is not the only cause of clotting defects. Compared with the criteria applied to HCCs, those used for METs are more restrictive, because metastatic tissue is more difficult to ablate than HCC tissue, so METs with diameters exceeding 2.5 to 3 cm are often considered ineligible for curative percutaneous ablation.
In terms of safety, one of the most important factors to consider is the location of the tumor. For nodules located superficially in segments III, IV, or V of the liver; on the subdiaphragmatic surface of the liver; and for exophytic nodules (surrounded by abdominal viscera) or those close to gallbladder, a laparoscopic or surgical approach is preferable, although in experienced hands, percutaneous ablation of these tumors is by no means impossible (Chopra et al, 2003). Caution must be used when the tumor is situated close to a major bile duct, because damage to these structures can lead to biliary stricture or fistula, which often requires additional interventional radiologic procedures or surgical correction (see Chapter 42A, Chapter 42B ).
For tumors located near a large hepatic blood vessel, risk of incomplete treatment and treatment failure is increased. Flow through these vessels can increase convectional heat loss near the tumor, thereby reducing the volume of the thermal lesion, and it can also result in more rapid clearance of the chemical ablative substances. It is often useful to devascularize these tumors before chemical or thermal ablation; this can be accomplished with selective transarterial embolization (TAE; see Chapter 83) or balloon-catheter occlusion of the vessels supplying or draining the tumor (Rossi et al, 2000).
Assessment of Local Tumor Control
Evaluation of local efficacy is based largely on evidence of complete tumor ablation that persists during the follow-up (lack of local recurrence). To achieve this, the entire tumor must be included in the volume of tissue that undergoes chemically or thermally induced coagulative necrosis, which renders it completely avascular. Shortly after the procedure, the necrotic area is surrounded by a halo of edematous, intensely hyperemic tissue. A few days later, this halo is replaced by an inflammatory halo, which persists for approximately 1 month. Thereafter, the mass of necrotic tissue shrinks and is replaced by fibrotic tissue (Rossi et al, 1990). The location, size, and evolution of this ablation zone are assessed with radiologic imaging techniques.
Evaluation of Immediate Treatment Response
The immediate response to any type of percutaneous ablation procedure is generally assessed with contrast-enhanced US (CEUS), enhanced spiral CT, or T2-weighted MRI—the gold standards for this purpose (Chen et al, 2007; Cioni et al, 2001). The presence at the ablation site of a well-defined, nonenhancing area as large or larger than the treated tumor itself is regarded as reliable evidence of a complete response, or complete radiologic necrosis. The timing of the imaging study is important. CEUS examinations performed too soon after ablation are characterized by numerous artifacts caused by gases produced during ablation or the diffusion of chemical substances within tissues surrounding tumor. Under these circumstances, it is impossible to distinguish the origin of the hyperechoic signals. If CEUS, CT, and MRI are performed in the days following the ablative procedures, it is impossible to determine whether the peripheral enhancement observed is due to residual tumor tissue or the hyperemic/inflammatory response of the liver parenchyma. This enhancement is a result of inflammatory tissue response, and it resolves within a month of tumor ablation. Therefore the persistence of an enhancing rim after this point must be considered evidence of residual tumor viability. For these reasons, the contrast-enhanced imaging studies should be scheduled at least 4 weeks after the procedure. If the results of this study are ambiguous, US-guided FNB can resolve the problem (Rossi et al, 1998).
Evaluation of Long-Term Results
Abdominal US/CEUS studies and tumor marker assays are performed every 4 to 6 months, or even more frequently if needed. Local recurrence is diagnosed when enhancement reappears within the ablation zone or within 2 cm, 1 cm according to some authors, from its margins, or when there is histologic evidence of tumor viability in this area (Berber et al, 2005). If the ablation zone remains unenhanced but fails to shrink during follow-up, or when levels of tumor markers are elevated in the absence of other intrahepatic or extrahepatic lesions, focused US-guided biopsy of the ablation zone is indicated. Nonlocal recurrence comprises extrahepatic metastases and all new intrahepatic regrowth located more than 2 cm from the ablation zone.
Assessment of Complications
Immediate and late complications have been reported after all types of percutaneous tumor ablation. They should be evaluated according to previously described guidelines (Sacks et al, 2003). Major complications are those that, if left untreated, could threaten the patient’s life, produce substantial morbidity, and/or prolong the hospital stay. All other events are considered minor complications. In general, patients treated with percutaneous ethanol injection (PEI)/percutaneous acetic acid injection (PAI) are monitored for 2 to 3 hours in a dedicated recovery room and are then discharged after a postablation abdominal US examination (Ebara et al, 1995; Shiina et al, 1991). Laboratory tests and other studies are indicated only when clinical or US evidence of complications are apparent. The 24-hour study is also accompanied by measurements of hemoglobin, lactic dehydrogenase and aminotransferases levels, and blood studies to assess hepatic function.
Chemical Ablation
Chemical ablation is based on the cytotoxic properties displayed by certain chemical substances when directly injected into a tissue. The most widely used cytotoxic substance is ethanol, but in some centers, acetic acid has recently been used as an alternative in the treatment of HCCs (Huo et al, 2003), and sodium hydroxide is under evaluation in a tumor model after evaluation in rats (Lin et al, 2001). Chemical ablation has been extensively used to treat HCCs. It has also been evaluated for the treatment of METs, but this experience was abandoned based on unsatisfactory results (Livraghi et al, 1991).
Percutaneous Ethanol Injection
Physical Principles
Direct injection of ethanol into a tumor results in its distribution in the tissue by diffusion and convection. Diffusion is favored by the existence of a concentration gradient between the injection point and the surrounding tissues, by the low molecular weight of the ethanol, by the increase in local pressure determined by the injection, and by the hypervascularity of the tumor (Ho et al, 2007). Ethanol spreads through the tumor tissue, exerting cytotoxic effects that include cytoplasmic dehydration and denaturation of cellular proteins. The endothelial cell necrosis and platelet aggregation it causes occlude tumor vessels and produce ischemic damage in the neoplastic tissue (Shiina et al, 1991; Ebara et al, 1995). The final result is coagulative necrosis within the tissue around the site of injection.
Technique
The procedure is performed under real-time US monitoring. The ethanol is usually injected with a 20- to 22-gauge endhole needle (Chiba or Chiba-like) or a conical-tip needle with multiple sideholes (Livraghi et al, 1995). The tip is positioned at the desired point in the HCC nodule with the aid of real-time US guidance, and the predetermined amount of ethanol is infused slowly into the tumor. After few seconds, the HCC nodule becomes completely hyperechoic. Light aspiration is applied during withdrawal to prevent excess ethanol from leaking into the peritoneum through the insertion tract. If all goes well, the patient is kept under observation for about 2 hours.
The volume of ethanol required to ablate a tumor nodule depends on the size of the tumor, but the final decision on the total dose is based on the results of imaging studies performed at the end of each session (Livraghi et al, 1995; Shiina et al, 1991).
An alternative to this multiple-session approach is one-shot PEI. As the term implies, the total amount of ethanol required for the ablation, based on the calculations reported above, is injected during a single session (Giorgio et al, 1996; Livraghi et al, 1993). This technique has been proposed for HCCs with diameters exceeding 3 cm. It is more painful than conventional PEI and is therefore performed under general anesthesia. In one study, the amounts of ethanol administered with this technique ranged from 20 to 165 mL (mean, 62 mL).
Percutaneous Acetic Acid Injection
Physical Principles and Technique
After ethanol, the most widely used agent for chemical ablation is acetic acid. In vitro studies have shown that it dissolves lipids and extracts collagen from various kinds of tissues (Ho et al, 2007). Compared with ethanol, acetic acid offers certain advantages, including more effective destruction of tumor septa and capsules. In addition, the same degree of cell kill can be obtained with a smaller infusion volume (Ohnishi, 1998).
The technique of PAI is quite similar to that of PEI. The infusion consists of a 50% solution of acetic acid and sterile water injected with the same technique and needles used for ethanol. Observations in animal models and clinical explants indicate that the volume of acetic acid needed to ablate a tumor nodule is roughly one third of the volume calculated with the formula used for ethanol: ablation of a nodule measuring 3 cm in diameter would require 34 mL of ethanol but only 11.3 mL of acetic acid (Giorgio et al, 2000).
Complications of Percutaneous Ethanol/Acetic Acid Injection
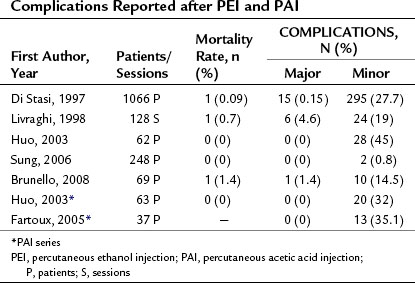
Although PEI and PAI are both low-risk procedures, in rare cases they can produce severe complications. Table 85A.1 shows the complication rates reported after these procedures. The mortality rates associated with PEI/PAI are very low, ranging from 0% to 1.4%. Major complications, which ranged from 0% to 4.6%, include hemoperitoneum, hemobilia, subcapsular or intraparenchymal hematoma, hepatic abscess, cholangitis, intestinal perforation, hepatic venous thrombosis, pneumothorax or pleural effusion, hepatic infarction, and tumor seeding along the needle insertion tract.
Local Tumor Control of Chemical Ablation
Several authors have analyzed the risk factors for local recurrence of HCC after PEI, and tumor size seems to be the most accurate predictor of this outcome. The 2-year local HCC recurrence rates reported after PEI are 10% for nodules smaller than 2 cm in diameter, 18% for those between 2.1 and 3 cm, and 30% for those larger than 3 cm (Ishii et al, 1996). In other studies, local recurrence rates ranged from 33% to 38% for HCCs smaller than 3 cm and from 43% to 68% for those exceeding 3 cm in diameter (Gaiani et al, 2003; Khan et al, 2000). Thus far, complete response rates observed after PAI seem to be similar to those obtained with PEI, but very few studies are available for comparison.
Long-Term Results
Several investigators have reported 5-year survival rates close to 50% after PEI for HCCs up to 3 cm in diameter in patients with cirrhosis; in other words, results are similar to those achieved with resection (Bruix & Llovet, 2002). In a consecutive series of 685 patients with HCC nodules measuring 3 cm or less, initial treatment with PEI was reportedly associated with a cumulative 5-year survival rate of 49% (Shiina et al, 2009). The Seventeenth Nationwide Follow-up Survey conducted by the Liver Cancer Study Group of Japan revealed survival rates of 91.3%, 77.5%, 63%, 50.2%, and 39.4% at 1, 2, 3, 4, and 5 years, respectively, for 14,726 patients whose HCCs were treated with ethanol injection (Ikai et al, 2007). A randomized trial failed to detect any significant difference in survival between HCC patients treated with PEI or surgical resection (Huang et al, 2005); however, other randomized trials have also shown that radiofrequency ablation (RFA) is superior to all types of chemical ablation for small HCCs, and the difference involved treatment responses, local tumor control, and survival rates (Bouza et al, 2009; Lin et al, 2005; Orlando et al, 2009; Shiina et al, 2005). For this reason, thermal ablation techniques are now preferred over chemical ablation for treatment of HCC. The chemical approach is still the treatment of choice when thermal ablation cannot be performed safely, such as when tumors form adhesions to the gastrointestinal tract, or when tumors are located in difficult sites or close to a bile duct.
Thermal Ablation
The goal of thermal ablation methods is to completely destroy a tumor without damaging the surrounding liver tissue. This can be achieved by generating killing temperatures, those exceeding 45° C or less than −40° C, within the mass of tissue that has to be ablated. The temperature reached and the duration of exposure determine how rapidly cell death occurs. Hyperthermia can be produced by delivering electromagnetic (radiofrequency, microwave), light (laser; see Chapters 85C and 85D), or mechanical (high-intensity focused ultrasound) energy into the tissue by means of dedicated probes. Hypothermia is created with cryoprobes (see Chapter 85B), which subtract heat from the tumor tissue by convection. RFA is the thermal technique most widely used for HCCs and liver METs and the one supported by the largest body of published evidence. Microwave ablation (MWA) and laser thermal ablation (LTA) have also been used in these settings, but these experiences are more limited. To date, experiences with high-intensity focused ultrasound (HIFU) have been confined to experimental studies or preliminary clinical investigations.
Radiofrequency Ablation (see Chapter 85C)
Physical Principles
Radiofrequency (RF) energy generates heat in the tissue that is in direct contact with the noninsulated tip of the needle electrode (McGahan et al, 1990; Rossi et al, 1990). The heat is the result of ionic and molecular friction, and it spreads into the surrounding tissues by a process of conduction. The highest temperatures are created in the tissue immediately adjacent to the electrode tip, and the heat rapidly decreases as the distance from the tip increases. Permanent tissue destruction occurs at temperatures of 45° C or higher (Cosman et al, 1983; Organ, 1976). Temperatures ranging from 46° C to 60° C produce irreversible cellular damage only after relatively long periods of exposure. In contrast, temperatures between 60° C and 100° C cause almost instantaneous protein coagulation with irreversible damage to mitochondria and cytosolic cell enzymes. When temperatures exceed 100° C, tissue fluids undergo boiling, vaporization, and ultimately carbonization. The final size of the thermal lesion, an area of coagulative necrosis that is gradually replaced by fibrotic tissue, depends on the total amount of heat deposited in the tissue, the thermal and electrical conductivity of the tissue, and the amount of heat lost through convection; specifically, it depends on heat lost through local blood flow, which acts as a heat sink. Tissue impedance limits the amount of heat that can be introduced into a tissue. It is inversely related to tissue hydration, which in turn reflects the ion content of the tissue (Djavan et al, 1997). During RFA the ions are quickly destroyed, and the tissue undergoes desiccation and charring, and the resultant increases in impedance ultimately prevent further delivery of RF energy.
Conventional RF electrodes used in the monopolar mode produce thermal lesions with a maximum diameter of about 1.8 cm (Rossi et al, 1996). Consequently, the ablation of a relatively small HCC nodule requires the creation of multiple, overlapping lesions. This used to mean multiple needle insertions and multiple treatment sessions; however, second-generation RF electrodes produced thermal lesions with diameters as large as 3 cm, and the volume of tissue that could be ablated with a single pulse of energy increased exponentially. These advances were achieved by increasing the active surface area of the electrode with a set of retractable hooks or prongs (expandable-tip electrodes; Rossi et al, 1998) or by cooling the electrode tip by means of an internal water-circulation system (cooled-tip electrodes) (Goldberg et al, 1998b). The expandable electrodes allow the delivery of larger amounts of RF energy, because rapid charring is less likely when the volume of tissue to be dehydrated is large. Cooled-tip electrodes achieve the same result by preventing temperatures rising above 100° C in tissues adjacent to the active surface of the electrode. The heat dissipation prevents charring and keeps impedance low, thereby allowing the delivery of larger amounts of RF energy. Larger thermal lesions, those 4 cm or larger, have also been produced with multiple cooled electrodes combined in a cluster (Head & Dodd, 2004), cooled catheter electrodes, and expandable spiral electrodes (Rossi et al, 2006).
Other investigators have attempted to increase thermal lesion volumes by reducing convectional heat loss during the RF procedure (Goldberg, 1998a; Patterson et al, 1998; Rossi, 1999). HCCs are supplied almost exclusively by vessels arising from the hepatic artery (Breedis & Young, 1954); however, when RFA was performed after balloon-catheter occlusion of the hepatic artery, the thermal lesions produced were large as expected on the basis of experimental studies; when RFA was performed after embolization of the feeding arteries with gelatin sponge particles, the thermal lesions produced were larger than expected (Rossi et al, 2000). With this approach, in fact, HCC nodules more than 6 cm in diameter could be treated in a single RF session.
Low impedance during the procedure explains the unexpectedly large thermal lesions. Occlusion of the tumor’s arterial supply with gelatin sponge particles reduces impedance in at least two ways: First, it increases the hydrostatic pressure within the HCC (Rossi et al, 2007). As a result, the boiling point of tissue fluid is higher during the thermal ablation procedure, which prolongs the delivery time and allows more energy to be deposited in the tissue, before desiccation and charring occur. Second and more importantly, a gelatin sponge is a hydrogel capable of modifying the electrical and thermal conductivity of the tissue; its presence in the ablation area markedly increases the amount of RF energy that can be delivered.
Technique
Patients undergoing RFA are hospitalized and treated after an overnight fast. In our department, most RFA procedures are performed without general anesthesia or conscious sedation, but some investigators prefer to perform the procedure under general anesthesia or deep sedation. A grounding pad is attached to the patient’s back and connected to the RF generator to close the electrical circuit. A local anesthetic (1% lidocaine) is injected along the predefined electrode insertion line, from the skin to the peritoneum. The skin is nicked with a small lancet to facilitate insertion of the electrode, and the tip is then advanced into the HCC nodule under real-time US guidance. The RF generator is activated, and the predefined amount of energy is delivered for 8 to 12 minutes. On US, the nodule becomes hyperechoic with a posterior acoustic shadow (Rossi et al, 1996). The pull-back technique can be used to create multiple thermal lesions along the major electrode axis (Rossi et al, 2000). At the end of the procedure, the electrode is withdrawn, but the generator remains on during this phase; this way, the electrode tract itself also undergoes coagulation, which diminishes the risks of bleeding and tumor seeding.
This technique can be used for HCCs up to 3.5 cm in diameter and for CRC liver METs with diameters of 2.5 cm or less. For larger tumors, other treatment strategies have been adopted. For HCC nodules whose diameters exceed 3.5 cm, RFA has been performed with a multiple-insertion technique (Livraghi et al, 2000), an expandable triple-spiral electrode (Rossi et al, 2006), clusters of cooled electrodes (Cheng et al, 2008), and combination of RFA with other techniques (Murakami et al, 2007). As discussed above, a larger volume of necrotic tissue can also be achieved by preablation embolization of the tumor with gelatin sponge particles. Some operators perform selective transarterial chemoembolization (TACE; see Chapter 83) of larger tumors after RFA debulking. The rationale of this approach is that the amount of embolic agent will be greater in the tumor tissue if its volume was previously reduced with RFA.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree