Percutaneous Endourology & Ureterorenoscopy: Introduction
Retrograde instrumentation of the upper urinary tract, such as ureterorenoscopy, uses under endoscopic guidance the anatomically predestined access via the urethra and ureter, while techniques of antegrade instrumentation require a percutaneous puncture. This approach must respect the intrarenal anatomy just as in open surgical nephrotomy, and imaging techniques are required to guide the procedure.
First, and most important, a puncture direction must be determined that will provide straight access to the target and safe, bloodless instrumentation. Visualization of both the puncture needle and the target and precise guidance of the needle tip to the target require imaging techniques such as ultrasound, fluoroscopy, and, in selected cases, computed tomography (CT).
Contraindications to percutaneous kidney puncture are blood-clotting anomalies due to coagulopathies or pharmacologic anticoagulation. Preparation and draping of the surgical field are required as for open surgery, and the same standards of asepsis must be followed. Local anesthesia only is sufficient for puncture of the kidney and small-bore tract dilation (6–12F), for antegrade insertion of a ureteral stent or nephrostomy catheter. Lidocaine hydrochloride 2% USP, 10 mL, can be given for infiltration of the skin and tissues along the intended tract of puncture down to the renal capsule. During dilation of the tract, administration of a local anesthetic in lubricant (eg, lidocaine hydrochloride jelly 2%) serves the dual purpose of anesthetization and lubrication. Dilation of nephrostomy tracts up to 30F and extraction of small renal stones can be done under local anesthesia.
Percutaneous nephrolithotomy (PNL) remains indicated for treatment of large stones, staghorn calculi, and stones in caliceal diverticula. The extent of intrarenal instrumentation for stone disintegration and extraction usually requires epidural or general anesthesia. Because puncture, tract dilation, and stone disintegration and removal are preferably performed as a one-stage procedure, the use of local anesthesia in PNL is limited.
Imaging and Puncture Techniques
Percutaneous puncture of the renal collecting system may be performed for diagnostic procedures (eg, antegrade pyelography, pressure/perfusion studies) or to establish access for therapeutic interventions (Table 8–1).
Diagnostic indications |
Antegrade pyelography |
Pressure/perfusion study (Whitaker test) |
Therapeutic indications |
Nephrostomy catheter drainage |
Antegrade ureteral stenting |
Dilation of ureteral strictures |
Percutaneous endopyeloplasty |
PNL |
Percutaneous resection and coagulation of urothelial tumors |
Both ultrasonic scanning and fluoroscopy provide visualization and guidance for a safe, accurate percutaneous puncture, but ultrasound has the following definite advantages:
No intravenous or retrograde administration of contrast dye
No radiation exposure
Continuous real-time control of puncture
Imaging of radiolucent, non–contrast-enhancing renal and extrarenal structures (eg, renal cyst, retroperitoneal tumor) for puncture
Imaging of all tissues along an intended nephrostomy tract (eg, bowel, lung)
Imaging in numerous planes simply by shifting, tilting, and rotating the scanning head
Three-dimensional information during puncture
Once the puncture needle has entered the renal collecting system, fluoroscopy is required for control and guidance of subsequent steps (eg, guidewire insertion, tract dilation, and catheter insertion). In selected cases, insertion and placement of a nephrostomy catheter in a dilated renal system may be possible with ultrasonic control only. Fluoroscopy provides a two-dimensional summation image with integration of all information from the third (anterior-posterior) dimension so that the entire length of a radiopaque catheter, wire, and so on can be visualized.
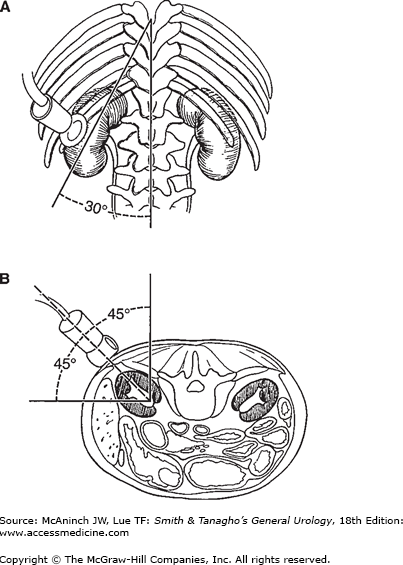
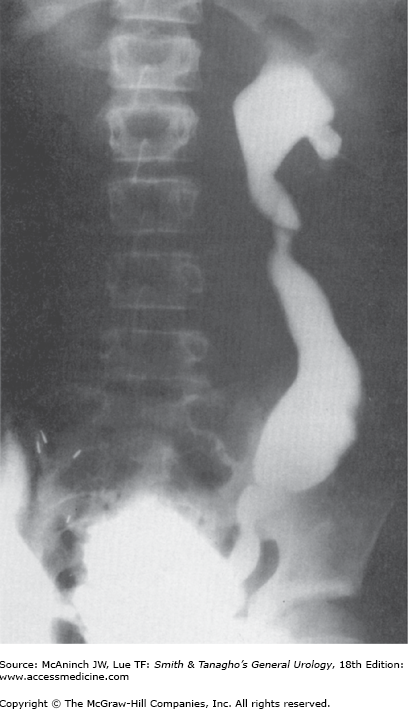
For percutaneous puncture of the renal collecting system, the patient should be placed on the fluoroscopy table in the prone position. Radiolucent bolsters may be placed under the abdomen to correct for lumbar lordosis and to support the kidney. A standard puncture site is in the posterior axillary line midway between the 12th rib and the ileal crest; this site ensures that later the patient does not lie on the nephrostomy catheter in the supine position. Morbidly obese patients can be placed in a lateral decubitus position in order to minimize respiratory distress. Ultrasonic scanning is performed below the 12th rib to obtain a median longitudinal scan through the kidney. For optimal coupling of the ultrasonic beam to the skin, sterile gel (eg, K-Y jelly) is applied to the skin at the scanning site. In the frontal view of an intravenous pyelogram, the long axis of the kidney usually follows the psoas muscle, forming about a 30° angle with the midline (Figure 8–1A). In the transverse view of a CT scan, the transverse axis of the kidney forms about a 45° angle with both a horizontal and a sagittal line (Figure 8–1B). The position and direction of the transducer should be oriented roughly to the following marks: below the 12th rib (if possible), cranial to the puncture site, with a 30° caudal-lateral rotation, and with a 45° lateral tilt of the scanning head.
Factors that may influence the choice of scanning technique and puncture site include patient size; position and rotation of the kidney; anomalies of bony structures; positions of the colon, spleen, liver, and lung relative to the kidney; and the target of puncture (upper, middle, or lower calyx; caliceal diverticulum). The scanning head can be positioned to provide the best visualization and optimum puncture site for each patient. Thus, a puncture site as high as above the 11th rib may be chosen if the lung is not visualized in the puncture direction. A different puncture site must be chosen if bowel gas or the liver or spleen is visualized within the intended puncture direction.
The puncture direction should always aim through a pyramid into a dorsal calyx; puncture into an infundibulum may result in bleeding from segmental and interlobar vessels in the renal sinus, and direct puncture of the renal pelvis renders dilation of the nephrostomy tract and insertion of catheters and instruments difficult, with increased risk of accidental catheter displacement after successful entry. For large, complete staghorn calculi, when PNL is to be performed for debulking the stone volume (followed by extracorporeal shockwave lithotripsy [ESWL] for disintegrating retained caliceal stones), puncture is usually performed through a lower dorsal calyx, a position from which the lower caliceal group, the renal pelvis, and part of the upper caliceal group can be reached easily with rigid instruments. However, for staghorn stones that are intended to be removed by PNL only (without ESWL), another access (eg, middle or upper calyx puncture) may be chosen. Stones in caliceal diverticula are approached by direct puncture of the diverticulum.
Once chosen, the target for access to the renal collecting system must be visualized ultrasonically. The cutaneous puncture site should be chosen in a virtual caudal extension of the perpendicular orientation (width) of the scanning plane. Skin and fascia are incised with a no. 11 blade. At this time, the scanning head may be shifted over the incision to measure the exact distance between the incision and the target. A 16- to 18-gauge puncture needle (Figure 8–2) may then be inserted blindly through the incision and aimed in the direction previously determined by ultrasound. However, the needle should never be advanced blindly farther than through the abdominal fascia.
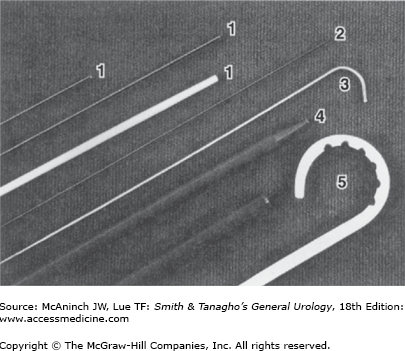
Figure 8–2.
Universal nephrostomy set (Bard-Angiomed), containing (1) coaxial 17.5-gauge needle with obturator/6F plastic sheath; (2) fine needle (22 gauge); (3) 0.035-in stiff guidewire with floppy J-tip (Lunderquist); (4) coaxial 10F dilator/12F introducer sheath system; and (5) 10F pigtail nephrostomy catheter.
The scanning head is now placed in such a way that both the target and the puncture needle are visualized in the same scanning plane, and the needle is aligned so that its tip can be clearly seen. Vibrating the needle makes the tip more visible while the position of the scanning head is being adjusted. The needle can be safely moved back and forth down to the renal capsule as often as necessary, but the renal parenchyma ideally should be punctured only once.
A needle guide attached to the scanning head can be used to direct the needle exactly within the ultrasonic scanning plane. With some needle guides, the angle of puncture relative to the longitudinal axis of the scanning plane (depth) is also fixed and is shown on the monitor by an electronically generated line. If a steeper or flatter angle of puncture is desired, the entire scanning head and attached needle guide must be tilted, and the choice of puncture site is therefore limited. Another drawback of a needle guide is that it does not allow for independent adjustment of the puncture and scanning direction if the needle deviates from its intended direction after being advanced through the skin. This frequently occurs in patients with scars from previous operations and becomes more of a problem the farther the target is from the cutaneous puncture site. Freehand puncture with individual adjustment of puncture and scanning direction is preferable in these cases.
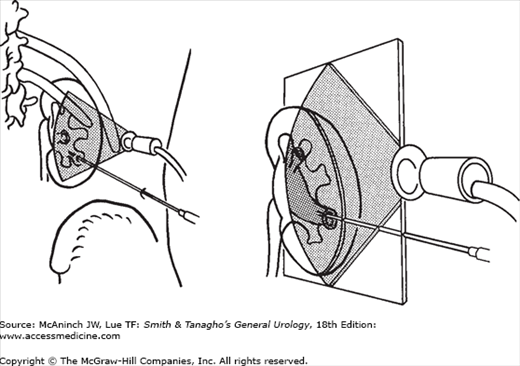
Movement of the kidney during respiration may complicate puncture if the target is small and is visible on the monitor only during a specific respiratory phase. If the direction of the needle and the position of the target are aligned and both are clearly seen on the monitor, the needle is advanced through the renal capsule during the appropriate phase of respiration (Figure 8–3). In this phase, the kidney is usually pushed to some extent by the puncture needle so that visualization of needle and target may be momentarily impaired. However, as soon as the tip of the needle has penetrated the fibrous renal capsule, it is seen even more clearly. If both the tip of the needle and the target are visualized clearly at the same spot on the scanning plane, the needle is in the desired space.
Antegrade injection of a small amount of contrast dye for fluoroscopy outlines the renal collecting system after successful puncture. However, if the collecting system has not been successfully punctured at the first attempt, contrast dye may fill the interlobar veins, which form a basket-like structure around the calyx, or may extravasate. In rare cases in which contrast dye is injected into the adventitia of the renal collecting system, extravasation may assume the configuration of the collecting system, mimicking successful puncture. Care must be taken to inject the least amount of dye necessary so that further fluoroscopic and ultrasonic orientation will not be hindered. A larger amount of dye injected outside the collecting system may compress the calyx to be entered and render puncture more difficult. If the position of the needle tip on ultrasound is close to its destination (ascertained by a small vibratory movement), the needle should be retracted a few millimeters only and readvanced at the appropriate angle and tilt. Once the collecting system is entered (Figure 8–4A), fluoroscopy alone is used to guide the subsequent steps of the procedure.
If fluoroscopy is used instead of ultrasound for guiding renal puncture, a fine-needle (20–22 gauge) puncture technique may be used. Intravenous or retrograde administration of contrast dye is needed. With retrograde injection, a ureteral balloon occlusion catheter can be inserted and blocked in the ureteropelvic junction (UPJ) to cause slight distention of the renal collecting system; this facilitates puncture of a nondilated system. First, a 16- to 18-gauge needle is inserted through the abdominal wall only, and a longer fine needle is inserted coaxially through the larger needle (Figure 8–4B). This technique improves control of the fine needle. As soon as the fine needle has entered the collecting system, the larger needle can be advanced over the fine needle, which serves as a guide. After withdrawal of the fine needle, a regular guidewire can be inserted through the large needle into the collecting system.
Antegrade Pyelography and Pressure/Perfusion Studies
Renal puncture is rarely indicated for diagnostic antegrade pyelography only, because less invasive radiographic techniques are available (eg, intravenous pyelography, ultrasound, CT, magnetic resonance imaging [MRI], retrograde pyelography). However, obtaining a radiograph after antegrade injection of contrast dye should be an integral part of every percutaneous puncture for any indication. Before contrast dye is injected, urine must be aspirated to decompress an obstructed collecting system. The contrast dye should be diluted to 20–30% for better visualization of details; antegrade pyelography then provides images of the collecting system with about the same resolution of detail as retrograde pyelography.
Antegrade pyelography is also performed in conjunction with a percutaneous pressure/perfusion study (Whitaker test) to assess pyeloureteral resistance. Percutaneous urodynamic studies of the dilated upper urinary tract are indicated only in the 10–30% of cases in which noninvasive radioisotope studies (diuresis renogram) fail to differentiate an obstructed dilated system from a nonobstructed dilated system. (This is more likely in cases of ureterovesical obstruction than in pelvic–ureteral obstruction, in which diuresis renograms are reliable.)
The Whitaker test provides simultaneous measurements of intrapelvic and intravesical pressures during antegrade perfusion, with flow rates of 5, 10, 15, and 20 mL/min. Puncture of the renal collecting system is performed with a coaxial needle/catheter system with an outer 6F catheter for the renal pressure/perfusion study; thus, puncture and catheter insertion can be done as a one-step procedure. Perfusion is started with flow rates of 5–10 mL/min until steady-state equilibrium of pressure readings is reached and the entire upper urinary tract is opacified (Figure 8–5). Pressure readings may be obtained intermittently from the perfusion catheter via a three-way stopcock, or continuously, if a double-lumen nephrostomy catheter or two separate catheters for perfusion and pressure measurement are used. Continuous recordings during perfusion from a single-lumen perfusion catheter via a T connection yield erroneous pressure readings (the smaller the lumen of the nephrostomy catheter and the higher the perfusion rate, the higher the pressure reading), unless the resistance of the entire system was previously calibrated for each rate of perfusion. To obtain accurate pressure readings, the positions of the intrapelvic and intravesical pressure manometers must be adjusted to the level of the renal pelvis and bladder, respectively. At a flow rate of 10 mL/min, differential pressures (renal pelvic pressure minus bladder pressure) below 13 cm water are normal, between 14 and 22 cm water suggest mild obstruction, and above 22 cm water suggest moderate to severe obstruction. At flow rates of 15 and 20 mL/min, upper limits of normal pressure are 18 and 21 cm water, respectively.
Percutaneous Catheter Placement
Percutaneous nephrostomy catheter placement for drainage and decompression of the upper urinary tract is indicated if retrograde ureteral catheterization is not advisable (eg, in sepsis secondary to ureteral obstruction) or proves to be impossible (eg, impassable ureteral obstruction due to stone, tumor, or stricture). After percutaneous endourologic procedures, a nephrostomy catheter is usually left indwelling for a few days. To convert a nephrostomy catheter diversion into internal stent drainage, antegrade ureteral stenting through the nephrostomy tract may be attempted even in cases in which previous attempts at retrograde stenting have failed. The antegrade approach to stenting can be expected to be successful if failure of retrograde stenting was not related to mere mechanical ureteral obstruction but rather to ureteral tortuosity, false passage (ureterovaginal fistula, urinoma after open surgery), or inability to identify the orifice endoscopically (ureterointestinal anastomosis).
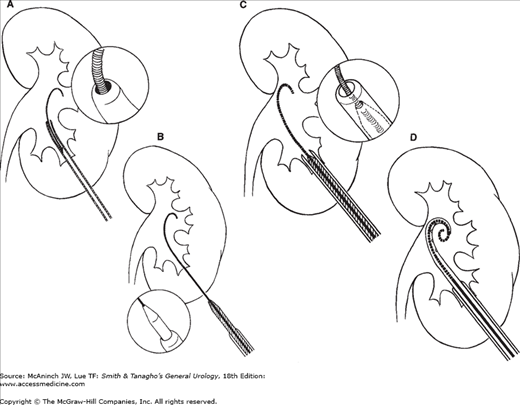
For diagnostic procedures such as pressure/perfusion studies (Whitaker test), a 6F catheter is sufficient. Catheters of this size can be placed in a one-step procedure of puncture if coaxial needle/catheter systems are used (Figure 8–2). For therapeutic interventions such as nephrostomy drainage or antegrade ureteral stenting, softer, larger catheters must be inserted, and puncture tract dilation is necessary before catheter insertion. For dilation of a puncture tract, a 0.035- or 0.038-in guidewire must be inserted into the collecting system, either directly through the puncture needle or through the outer catheter of a coaxial needle/catheter system. Curved-tip (J) guidewires are less likely to cause damage to the mucosa of the renal pelvis than are straight guidewires. One of the most common problems of tract dilation is kinking of the guidewire during insertion of fascial dilators; therefore, guidewires with a floppy tip and a stiff proximal section (Lunderquist wire) are preferable over floppy guidewires. If the tip of the guidewire cannot be advanced into the renal pelvis because it is trapped in a dilated calyx with a narrow infundibulum or because an obstructing stone hinders passage, the outer catheter of a coaxial needle/catheter system can be used to manipulate the guidewire into the collecting system (Figure 8–6A), or angiographic catheters with different curved-tip configurations may be inserted over the guidewire for this purpose. Once the guidewire is in the correct position (upper calyx, renal pelvis, upper ureter), radiopaque fascial dilators can be inserted under fluoroscopic control with rotating movement of the dilator during advancement. If flexible plastic fascial dilators are used, sequential insertion of dilators of increasing size (usually in 2F steps) is necessary. If stiff metal or Kevlar dilators are used, dilation from 6F to 10–12F is possible in a one-step procedure.
Figure 8–6.
Small-bore tract dilation and nephrostomy catheter insertion. A: J-guidewire inserted through the needle-sheath system and advanced with assistance of the plastic sheath into the renal pelvis. B: Insertion of a coaxial dilator/introducer sheath system over the guidewire. Stiff proximal section of the Lunderquist guidewire prevents extrarenal kinking. C: After the dilator has entered the collecting system, the introducer sheath is advanced over its tip. D: Pigtail nephrostomy catheter is inserted into the renal pelvis over the guidewire and through the introducer sheath.
After tract dilation, relatively stiff nephrostomy catheters (eg, polyethylene catheters) can be introduced easily over the guidewire. However, if softer catheters (eg, silicone or polyurethane catheters) are to be inserted, use of an introducer sheath is helpful. An introducer sheath is also helpful for antegrade ureteral stenting and for insertion of nephrostomy catheters with various self-retaining configurations of the tip (eg, pigtail). These catheters can be stretched into a straight configuration while being inserted through the introducer sheath and over a guidewire; the tip resumes its original configuration due to the memory function of the material once the guidewire is withdrawn. The introducer sheath can be inserted with the last fascial dilator in a one-step procedure if a coaxial dilator/introducer sheath system is used (Figures 8–6B and C). The use of an introducer sheath provides universal access to the renal collecting system for placement of all types of catheters (nephrostomy catheters [Figure 8–6D], ureteral stents, balloon dilation catheters) and safety and working wires for different systems of large-bore nephrostomy tract dilation required for insertion of endoscopic instruments.
Nephrostomy catheters should be soft to avoid discomfort and irritation of the renal pelvis and should have a self-retaining mechanism or should be placed with enough slack to prevent dislodgment from the collecting system during movement of the kidney. Standard nephrostomy catheters are Malecot catheters, pigtail catheters, and loop catheters. Loop catheters have a very effective retaining mechanism; they may cause serious complications, however, if the catheter is accidentally pulled out of the kidney.
Antegrade ureteral stenting can be done through the introducer sheath using either open- or closed-tip stents. Catheters with open-tip configuration are advanced with a pusher catheter over a guidewire, which must be inserted through the introducer sheath down the ureter and into the bladder as a first step. Catheters with closed-tip configuration are advanced by pushing the indwelling wire. In either technique, a thread through the proximal side hole of the catheter ensures that the catheter can be pulled back into the renal pelvis if it is advanced too far. The thread must be pulled out before the guidewire is withdrawn so that the pusher catheter can still hold the double-J stent in place.
An introducer sheath may also be used for insertion of a 7F balloon dilation catheter over a guidewire into the ureter to dilate ureteral strictures to 12–18F with balloon pressures of up to 15 atm. After successful dilation, an 8–10F stent is usually left indwelling for several weeks. This technique is most successful in ureteral strictures that are a complication of recent surgery for benign disorders, except ureteropelvic obstruction. Long-standing strictures or strictures due to tumor compression of the ureter, radiation damage, or ischemic ureteral necrosis after radical pelvic surgery are not likely to respond favorably to balloon dilation. Long-term results of ureteral balloon dilation cannot be determined from published data, either because the periods of follow-up were too short or because balloon dilations were repeated periodically.
Endoscopic Intrarenal Instrumentation
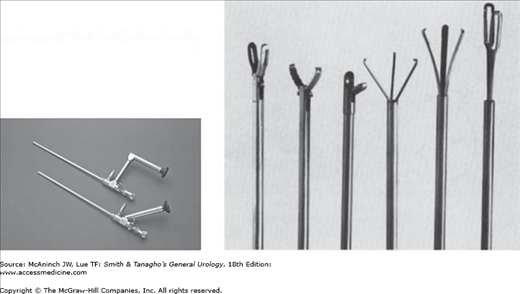
Nephroscopes are endoscopic instruments with sheaths of 15–26F that are inserted percutaneously through a nephrostomy tract. Standard rigid instruments are available in sizes 18–26F; these have telescopes with offset eyepieces (Figure 8–7, left). Rigid instruments such as graspers and ultrasound probes can be inserted through a central working channel (Figure 8–7, right). Flexible fiberoptic nephroscopes may be used as well. These have a deflecting mechanism for the tip that allows inspection of otherwise difficult-to-reach calyces. A smaller working channel allows insertion of flexible instruments such as stone baskets, wire graspers, and electrohydraulic or laser probes. However, instrumentation through flexible nephroscopes is limited by the size and flexibility of working instruments such as stone forceps, and flexible endoscopes do not offer the optical quality and durability of rigid nephroscopes.
Nephroscopy is rarely indicated for diagnostic purposes only; in most cases, it is performed for percutaneous lithotripsy and extraction of renal stones (PNL). However, ESWL has gradually replaced PNL for treatment of renal stones and is now used in >90% of cases. PNL is still indicated in cases for which ESWL is not the primary choice of treatment. Such cases include urinary obstruction not caused by the stone itself, large-volume stones, and stones that cannot be positioned within the focus of the shock wave apparatus. PNL can achieve stone-free rates of >90%. Nephroscopes also may be used for direct-vision internal incision of ureteropelvic stenosis and for endoscopic treatment of urothelial tumors of the upper urinary tract.
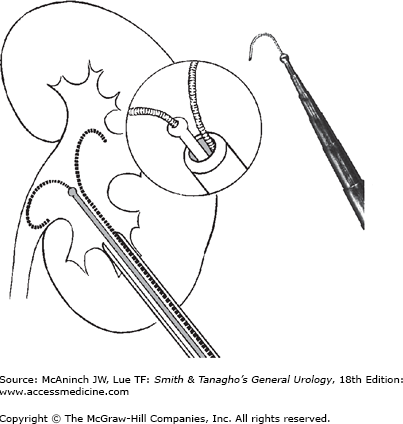
Insertion of a nephroscope into the renal collecting system requires dilation of the puncture tract to 18–30F. A safety wire should be inserted parallel to the working wire and advanced into an upper calyx or the upper ureter to guide the way back into the collecting system in case the dilator and working wire become displaced accidentally. Insertion of an introducer sheath during small-bore tract dilation to 10–12F facilitates parallel insertion of safety and working wires. The central metal catheter of a coaxial metal dilator system (Figure 8–8, left), the central plastic catheter for insertion of sequential plastic dilators, or a balloon dilator can be inserted over the working wire. Balloon dilators of 9F size can dilate a nephrostomy tract to a diameter of 30F under pressure up to 10–12 atm in a one-step procedure. This may prove difficult or impossible if perirenal scar tissue from previous surgery prevents complete expansion of the balloon over its entire length. Sequential plastic dilators allow stepwise dilation of the tract under fluoroscopic control; however, on withdrawal for insertion of the next larger dilator, compression of the tract is lost intermittently and bleeding occurs into the collecting system, sometimes hindering subsequent endoscopy. Reusable Alken coaxial metal dilators (Figure 8–8, right) (each dilator slides over the next smaller one) allow stepwise tract dilation even in the presence of severe scarring with continuous nephrostomy tract compression for improved hemostasis.
Figure 8–8.
Large-bore tract dilation for nephroscopy. Left: Insertion of the central catheter of the Alken dilator system over a working wire through the introducer sheath (see also Figure 8–6). An introducer sheath allows parallel insertion of a safety wire into the collecting system. Right: Alken coaxial metal dilators for sequential tract dilation without loss of tract compression. Final step is coaxial insertion of a plastic working sheath or the metal nephroscope sheath.
With any dilation technique, the last step is insertion of a working sheath, which can be either the metal working sheath of the nephroscope or a larger Amplatz plastic sheath. With the balloon dilation technique, the Amplatz working sheath must be introduced over a plastic dilator; with use of serial plastic or coaxial metal dilators, the working sheath slides over the last dilator. The Pathway Access Sheath is a balloon dilator with coaxial external expandable sheath. It allows for balloon tract dilation and percutaneous access sheath placement in one step. A 28–30F Amplatz plastic working sheath is preferable to the metal nephroscope sheath in all cases in which extensive, prolonged instrumentation is anticipated (eg, staghorn stones). Larger plastic sheaths not only provide better drainage of irrigation fluid with lower intrapelvic pressures than do continuous-flow nephroscope sheaths but also allow easier extraction of large stone fragments.
In the era of ESWL, indications for PNL are limited to four types of stone disease:
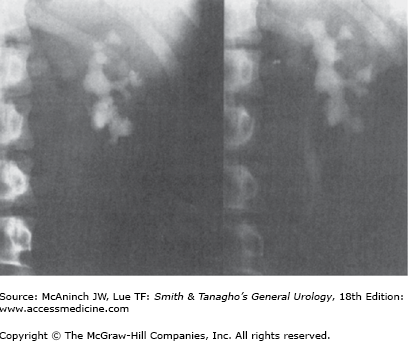
Urinary obstruction not caused by the stone itself (eg, stone in a caliceal diverticulum [Figure 8–9, left and right], stone in association with ureteropelvic stenosis). These stones could be broken up by ESWL, but gravel would not pass spontaneously.
Large-volume stones (>3 cm, stone surface >500 mm2) (Figure 8–10, left and right) (eg, staghorn stones). These stones can be treated by several sessions of ESWL, but only about 30% of patients become stone free. However, problems associated with passing large quantities of gravel (eg, ureteral obstruction, pain, fever, sepsis) can be prevented by first percutaneously debulking the stone and then performing ESWL for endoscopically inaccessible stones.
Stones that cannot be positioned within the focus of the shock wave apparatus (eg, stones in kidneys with abnormal position due to anomalies of the urinary tract or skeleton, stones in transplanted kidneys, kidney stones in very obese patients that cannot be positioned into the focus of the shock wave source due to the increased distance from skin to stone, or when the weight limit of the ESWL table is exceeded).
PNL may be of benefit for lower pole caliceal calculi even under the 2–3-cm range. The overall stone-free rate for these stones with ESWL is only about 60%.
Large-volume staghorn stones are a much more common indication for PNL than stones that can be extracted in toto. Small stones can be extracted with a variety of rigid forceps and graspers (Figure 8–7, right). Stones may be retrieved from difficult-to-reach calyces with flexible wire baskets and graspers inserted through flexible nephroscopes. Large stones must be disintegrated using mechanical, ultrasonic, electrohydraulic, or laser energy. Hollow ultrasonic probes allow for controllable, systematic stone disintegration under continuous suction for removal of sand and smaller fragments. Electrohydraulic probes are more powerful than ultrasonic probes and may be used through flexible nephroscopes, but they do not provide continuous suction and are associated with a higher risk of scattering stone fragments into inaccessible calyces and of damaging the mucosa of the renal pelvis. However, with electrohydraulic probes and the holmium: YAG laser, disintegration of hard or large stones is faster.
For soft stones, continuous disintegration and evacuation of fragments with ultrasound probes is most time efficient. Hard stones should be broken up into the largest possible fragments that can be extracted through the working sheath. The ureteropelvic portion of a staghorn stone should be left in place until the procedure is nearly completed, as it will act like a plug in a drain to prevent the loss of fragments into the ureter. An antegradely or retrogradely positioned ureteral balloon occlusion catheter might serve the same purpose; however, the extra procedure of retrograde ureteral catheterization is rarely indicated.
Normal saline should be used as the irrigation fluid except in the case of electrohydraulic lithotripsy, in which one-sixth normal saline is more appropriate. However, even with the low-pressure system provided by a large plastic working sheath, considerable amounts of irrigation fluid may be absorbed if small veins are opened and intrarenal manipulation is prolonged. This may cause transurethral resection (TUR) syndrome with use of hypotonic fluids. Intraoperative administration of diuretics (eg, mannitol, 12.5 g) is advisable and also has proved effective in preventing intrarenal reflux. If there is suspicion of extravasation, contrast dye must be injected and a diagnostic radiograph obtained. On completion of the procedure, a plain film should be obtained and a nephrostomy catheter placed. A Foley catheter with a 5-mL balloon may be inserted through a fenestrated trocar or through the plastic working sheath, which then is withdrawn and cut lengthwise for removal from the Foley catheter. Malecot catheters or straight polyethylene catheters (eg, chest tubes) may be used as well and should be secured to the skin with sutures. A final nephrostogram documents appropriate position of the catheter.
Nephrostomy catheters may be removed 1–4 days after antegrade pyelography to check for unobstructed upper urinary tract drainage. In cases of postoperative profuse hemorrhage, the nephrostomy catheter can be occluded for 1–2 days to allow for tamponade formation. Blood clots usually dissolve later spontaneously due to urokinase activity without problems. If ESWL is to be performed, it can be done 1–4 days after the percutaneous procedure. The nephrostomy catheter should be left in place during and after ESWL to provide drainage for urine and stone gravel and to allow for a second endoscopic procedure if some of the stone fragments do not pass spontaneously after ESWL.
After removing a large bore nephrostomy catheter (22–24F), urinary discharge from the nephrostomy tract can persist for several days and be bothersome and concerning to the patient. To prevent this, the nephrostomy tube can be exchanged over a guidewire under fluoroscopic guidance for a smaller caliber catheter. Leaving this smaller tube in place for a few days will permit the tissues around the tract to contract and minimize leakage.
Some experienced endourologists have propagated percutaneous stone treatment and endopyelotomy without standard placement of a nephrostomy catheter. Major advantages are marked reduction in analgesia requirements and length of hospitalization. Prerequisites are a small to moderate stone burden and no residual fragments, not more than two percutaneous tracts in one session, and no significant bleeding. Bleeding arising from the nephrostomy tract can be stopped nephroscopically by punctual electrocoagulation during withdrawal of the working sheath. However, this “tubeless” percutaneous renal surgery is best performed with intraoperative placement of a double-J ureteral stent to secure unobstructed urinary drainage. Expected patient discomfort from the nephrostomy catheter is traded against possible discomfort from the double-J ureteral stent and from cystoscopy to remove the stent later.