Fig. 19.1
(a, b) Digitally enhanced MRI reconstruction of the levator ani at rest in a 23-year-old nulliparous woman. The levator ani gives a biconcave shape posteriorly and the puborectalis show as a sling imbedded in the muscle of the pelvic floor (Reprinted with permission from Cleveland Clinic Center for Medical Art and Photography© 2004–2009 and Matthew Barber, MD)
The endopelvic fascia is found between the visceral peritoneum and parietal fascia of the levator ani and is a fibroareolar tissue containing neurovascular bundles, smooth muscles, collagen, and elastin (Fig. 19.2).
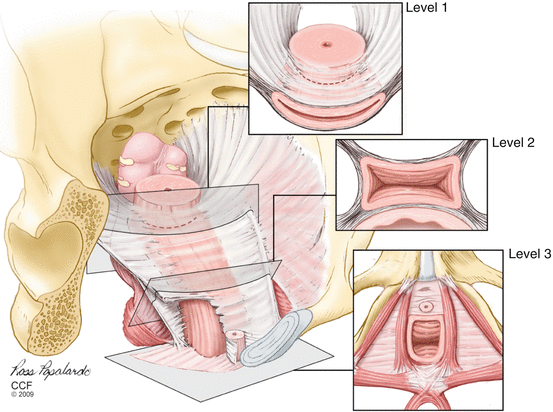

Fig. 19.2
Illustration of the endopelvic fascia as it fans out to cover the pelvic floor and provide support to the surrounding organs. The levels 1, 2, and 3 depict the vaginal support (Reprinted with permission from Cleveland Clinic Center for Medical Art and Photography© 2004–2009 and Matthew Barber, MD)
Equally important to the understanding of pelvic anatomy is an understanding of the dynamic changes of the pelvic organs. Kelvin and associates popularized the use of four-contrast study to outline the small bowel, bladder, vagina, and rectum. The dynamic evaluation of the pelvic floor before, during, and after evacuation of the contrasts in the rectum and bladder not only yields a tremendous amount of information about the function of the pelvic organs and the surrounding structures which support them, but it also complements physical examination for enterocele, one of the findings in advanced pelvic floor prolapse.
Kelvin et al., using their four-contrast study to evaluate 74 women with pelvic floor prolapse, found 14 (19 %) to have enteroceles, 50 % of which were missed on physical exam.
These dynamic studies also helped to identify other pelvic organ dysfunctions. In a study of 100 women referred for evaluation of pelvic floor prolapse, dynamic cystoproctography or cystodefecography found that of the 20 patients with anterior compartment systems (urinary), 45 % had middle compartment findings of vaginal vault prolapse; of posterior compartment findings, 90 % had rectocele, 40 % had enterocele, and 35 % had rectal intussusception.
Similarly, of the 45 patients with symptoms of middle compartment defects (genital), 91 % had anterior compartment findings of cystocele and 56 % of hypermobile bladder neck; of posterior compartment findings, 82 % had findings of rectocele and 58 % of enterocele.
Of the 17 patients with posterior compartment symptoms (anorectal), 71 % had cystocele, 65 % had hypermobile bladder neck, and 35 % had vaginal prolapse. Their study concluded that 95 % of the women with pelvic floor dysfunction had abnormalities of all three compartments.
This study underscores the global nature of pelvic floor disorders and the need to understand the pelvic floor as a unit rather than as compartments.
Enterocele
The loss of pelvic organ alignment as seen on cystodefecography of the bladder, vagina, uterus, and rectum is obvious, but the significance of an enterocele remains controversial.
Kinzel described an enterocele as a “true” hernia because it contains a hernia sac (the pouch of Douglas), neck, and contents.
Delancey postulates that enterocele sac develops as a result of the loss of suspension of the upper vagina and muscle integrity of the levator ani muscles that leads to the herniation of the cul-de-sac between the rectum and vagina.
Enteroceles are often classified as congenital, pulsion, traction, and iatrogenic.
A congenital enterocele is a result of the failure of the fusion of the anterior and posterior peritoneum during fetal development, resulting in a deep pouch of Douglas.
Pulsion type is caused by chronic increase of abdominal pressure.
While traction type is caused by the loss of pelvic floor support and resulting in the pulling or traction of the pelvic organ on the surrounding structures out of the pelvis such as the vagina.
Iatrogenic is caused by surgical injury.
The clinical presentations of an enterocele are dependent on the extent of the herniation and may present from no physical findings to bulging of the perineum or posterior vagina during strain.
Clinical examination of the vagina or bidigital exam of the rectum and vagina during maximum strain can help detect the spreading of the rectovaginal plane between the fingers as the enterocele enters into the rectovaginal plane; however, physical examination is unreliable in detecting enterocele.
Kelvin et al. reported their findings on 170 patients with symptoms of pelvic floor dysfunction who were evaluated by urogynecologist and found 47 patients (28 %) with an enterocele; only 24 (51 %) of these patients were found by physical exam.
There are, however, controversies about the significance of enterocele containing small bowel versus sigmoid colon.
Some contend that enteroceles containing sigmoid colon result in defecatory dysfunction, whereas enteroceles containing small bowel are more reflective of pelvic floor prolapse.
MRI has been reported to be useful in the detection of enteroceles (Fig. 19.3).
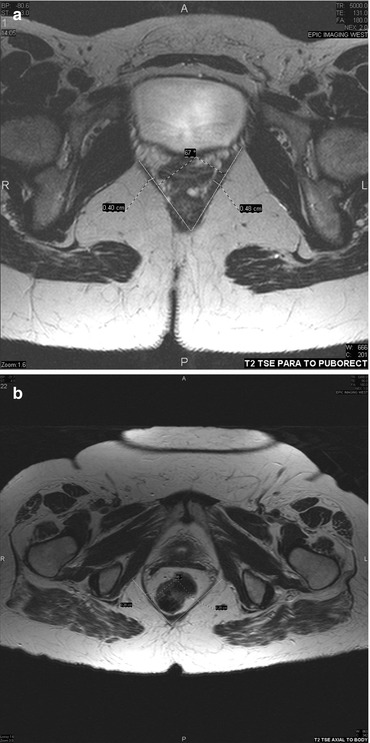
Fig. 19.3
MRI of the levator hiatus, normal on the top (a) and enlarged on the bottom (b). The angle between the two lines drawn along the puborectalis muscle and bisecting in the posterior midline of the levator ani defines the levator hiatus angle. This angle can also be measured by endorectal ultrasound and is found to correlate with findings of enterocele and pelvic floor prolapse findings on MRI and cystodefecography
It is also helpful in evaluating not only the size of the levator hiatus in prolapse but also the changes in the anatomy of the levator ani muscle.
Singh et al. used a three-dimensional MRI reconstruction to assess the size of the levator hiatus and morphology of the levator ani muscle.
They found that increasing stage of prolapse correlated with increasing size of the levator hiatus, but not with the morphology of the levator ani (Fig. 19.4).
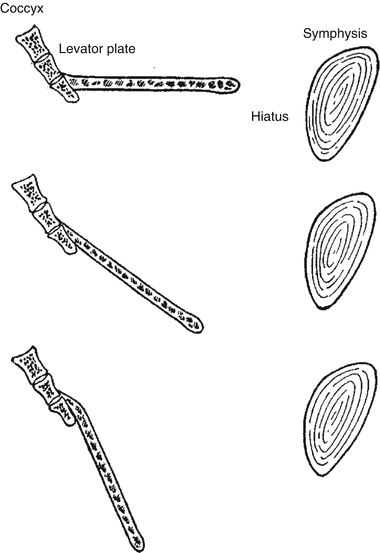
Fig. 19.4
Illustration of the incline of the levator plate and enlargement of the levator hiatus resulting in pelvic organ prolapse (With permission from Berglas B, Rubin IC. Study of the supportive structures of the uterus by levator myography. Surg Gynecol Obstet 1953;97:677–92)
They proposed that patients who have pelvic organ prolapse with normal levator ani morphology may only need fascial repair compared to those who show morphologic changes of muscle injury.
Currently, the MRI remains the best imaging technique in looking at the anatomy of the pelvic floor, while cystodefecography provides for dynamic images of the pelvic organs during defecation and urination.
The increasing use of open-configuration MRI to allow the patient to assume a sitting position has shown this to be equivalent to the supine MRI in the evaluation of pelvic floor laxity. In the future, the open-configuration MRI may offer superior imaging findings compared to cystodefecography.
The MRI and cystodefecography studies have helped confirm what is already clinically known about the enlargement of the levator hiatus in pelvic organ prolapse. What is not known is at what point in the levator hiatus enlargement does pelvic organ dysfunction or prolapse occur, and why.
The true incidence or prevalence of colorectal diseases associated with pelvic floor disorder is unknown.
This is in part due to our failure to recognize the early signs of pelvic floor laxity and in part due to the poor correlation between the degree of pelvic organ prolapse and symptoms.
In an attempt to identify patients with pelvic organ prolapse by history, Barber et al. found that when patients with high probability of prolapse were asked, “Do you usually have a bulge or something falling out that you can see or feel in your vagina?” an affirmative answer had a 96 % sensitivity (95 % CI 92–100) and 79 % specificity (95 % CI 77–92) for stage III and IV vaginal prolapse defined by the Pelvic Organ Prolapse Quantification (POP-Q).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






