Chapter 27 PATHOPHYSIOLOGY OF STRESS INCONTINENCE
The lower urinary tract provides continence by storing urine at low pressure until it is socially convenient and appropriate to void. This function is mediated by the presence of an expansible, low-pressure organ, the bladder, and a sphincter-controlled outlet mechanism. The outlet mechanism prevents urinary incontinence during an increase abdominal pressure by means of the sphincter and a complex pelvic support mechanism. Understanding the pathophysiology of stress incontinence at an anatomic level can help to identify specific anatomic defects and direct individualized treatment of patients suffering from incontinence.
THE STRESS CONTINENCE CONTROL SYSTEM
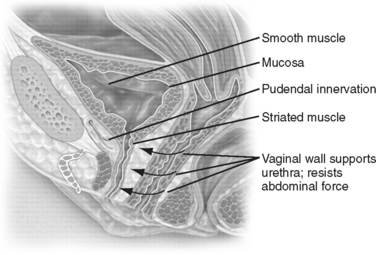
The urethral closure pressure must be greater than the bladder pressure at rest and during increases in abdominal pressure to maintain continence. The anatomic components necessary to meet this goal are a well-vascularized urethral mucosa and submucosa, a well-organized and functioning intrinsic urethral smooth muscle, a properly functioning striated sphincter with intact pudendal innervation (i.e., rhabdosphincter), and a stable, supportive hammock of surrounding muscular and fascial tissues (Fig. 27-1).
Intrinsic urethral function is the product of coaptation and compression along the length of the urethra, from the bladder neck to the proximal and mid-urethra.1 A robust anatomic support mechanism buttresses a competent bladder neck and functioning urethral mechanics and facilitates appropriate compression and pressure transmission during increases in abdominal pressure affecting the bladder outlet. These functional interactions are complex and remain the subject of ongoing research in terms of understanding mechanisms and developing measures of outlet function. Surgery directed at the bladder outlet continence mechanism at the level of bladder neck or mid-urethra aims to counteract the loss of urethral support by creating a new zone that offers support and acts as a back plate to absorb the transmitted pressure and safeguard the sphincteric configuration.
Urethral Sphincteric Mechanisms
The glandular secretions of the inner mucosa increase the surface tension, promoting its plasticity and increasing its ability to coapt.2 The abundant middle spongy vascular tissue of the submucosa forms a watertight seal and provides up to 30% of the total closure pressure3–5; this vascular cushion, like the other tissues of the urethra, is under the influence of estrogens.6–8 Estrogenic deficiency in postmenopausal women results in atrophy of this layer, reduces the hermetic seal of the urethra mucosal, and may contribute to the multifactorial cause of stress incontinence. Although there is some consensus among clinicians about the value of estrogen therapy for treating the symptoms of atrophic vaginitis in postmenopausal stress urinary incontinence, there is no evidence to support the use of exogenous estrogen replacement therapy in treating stress incontinence.9
The role of smooth muscle in the maintenance of female continence is still uncertain. The U-shaped loop of the detrusor smooth muscle surrounds the proximal urethra and favors its closure by creating a constant tone. Detrusor fibers at the bladder neck are longitudinally oriented, and they extend from the bladder neck to the subcutaneous adipose tissue that surrounds the urethral meatus. The inner longitudinal smooth muscle layer is surrounded by a circular layer that lies inside the outer layer of striated muscle. The smooth muscle layers are present throughout the upper four fifths of the urethra, and their circular disposition suggests that contraction has a role in constricting the lumen. The longitudinal smooth muscle does not seem to have a passive role during the filling; rather, it has been proposed that it acts by shortening and opening the lumen to initiate micturition.10
The striated urogenital sphincter consists of two parts, the rhabdosphincter and the compressor urethra and the urethrovaginal sphincter distally.11 The proximal one third of the urethra is surrounded by a sleeve of circular striated muscle that is continuous with a longer ascending cone, which extends to the vaginal introitus. The rhabdosphincter is composed mainly of type 1 fibers, composed of slow-twitch tissue capable of maintaining constant tonic contractions of the urethral lumen.12 From manometric and electrophysiologic evaluations, this produces the greatest level of resting pressure and electromyography activity.
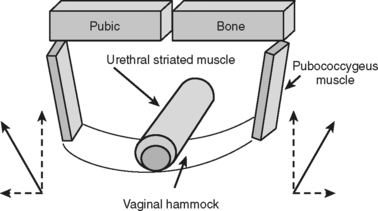
This part of the urethra is intrapelvic, immediately posterior to the pubic bone. In the past, it was suggested that when the urethra descended and lost this intra-abdominal position, intra-abdominal forces could no longer constrict it during straining, resulting in stress incontinence. This concept, the pressure transmission theory, which was introduced by Enhorning in 1961,13 evolved into the hammock theory of DeLancey14 (1994), which proposes that the vagina posteriorly offers a back plate against which the urethra is compressed during increases in intra-abdominal pressure. During a sudden increase in intra-abdominal pressure, such as during coughing, a reflex contraction of the pubococcygeus muscle occurs. The vaginal hammock is stretched and stiffened, making it easier for the urethral sphincter to close in a slitwise fashion against this firm back plate. The result and increase in intraurethral pressure, which is thought to be lost in patients suffering from stress incontinence, can be recreated by surgical procedures that stabilize or elevate the suburethral vaginal wall.15–18
If these postulates are correct, an appropriate contributory factor to female continence is provided by the bladder neck’s passive elastic tension, which is important in maintaining urethral closure.19,20 In the mid-urethra at rest, closure is mostly maintained by the intrinsic striated muscle of the rhabdosphincter; however, the extrinsic striated muscles of pubococcygeus and compressor urethrae may play a significant role in ensuring urethral closure during increases in intra-abdominal pressure. The plasticity of the urethra or its ability to work as a watertight seal is also important in maintaining continence. Approximately one third of the resting urethral pressure is offered by smooth muscle effects, one third by striated muscle effects, and one third by the vascular contribution.21
Urethral Support Systems
The urethral support mechanism comprises all the structures extrinsic to the urethra that offer a supportive back plate on which the proximal urethra and mid-urethra lie (Fig. 27-2). The anatomic support is derived more from the fascial structures than from the musculature itself. The pubourethral ligament attaches the mid-urethra to the inferior side of the pubic symphysis and prevents downward movement during its rotational descent.22 It works in conjunction with the pubourethralis muscle, a subdivision of the levator ani muscle that forms a sling around the proximal urethra. Together they form the mid-urethral complex. It has been postulated that elongation of the posterior pubourethral ligaments may be a significant contributory factor to the loss of urethral support seen in stress incontinence. Major fascial support to the urethra also is provided by the urethropelvic ligament, which attaches the urethra to the tendinous arc.23
The vesicopelvic fascia or pubocervical fascia, extending between the bladder and the vagina, suspends and attaches the vagina and cervix to the pelvic side wall, to each arcus tendineus fascia pelvis, thereby offering posterior support to the bladder and bladder neck. It has two surfaces: the perivesical fascia on the vaginal side and the endopelvic fascia on the abdominal side. Its upper zone supports the bladder above the cervix, the middle zone supports the trigone, and the lower zone supports the bladder neck. Laxity of the fascia in each of the zones results in uterine prolapse, cystocele, and urethrocele, respectively.24
The pelvic floor musculature, represented by the levator ani muscles, carries the weight of the pelvic contents and prevents the abdominal pressure from stretching the ligamentous support structures. The levator ani includes the puborectalis muscle, which surrounds the rectum, connecting the pubic bones anteriorly in a U-shaped configuration; the pubococcygeus muscle, which crosses from the pubis to the coccyx; and the iliococcygeus muscle. The iliococcygeus arises laterally from the arcus tendineus levator ani and forms a horizontal sheet that spans the posterior opening of the pelvis, providing a shelf on which the pelvic organs lie. The urethra and vagina pass through an aperture in the levator musculature, the urogenital hiatus. The constant muscle tone,25 maintained by predominantly type I (slow-twitch) striated muscle fibers, compresses the vagina and urethra anteriorly toward the pubic bone and keeps the hiatus closed.26
The pudendal nerve provides somatic innervation to the striated muscle of levator ani and to the striated muscle within the external anal and urethral sphincters. The intrapelvic somatic fibers travel along the anterior vaginal wall from the sacral segments S2 through S4 to provide a somatic nerve supply to the pelvic floor.27 Neuromuscular injuries have been proposed as an important factor in predisposing to pelvic floor dysfunction. Childbirth and age are considered the two major factors predisposing to pelvic floor denervation.28 Other factors include pelvic surgery (i.e., rectal and vaginal surgery with extensive pelvic dissection), radiotherapy, and congenital neurologic conditions such as spina bifida and muscular dystrophy.
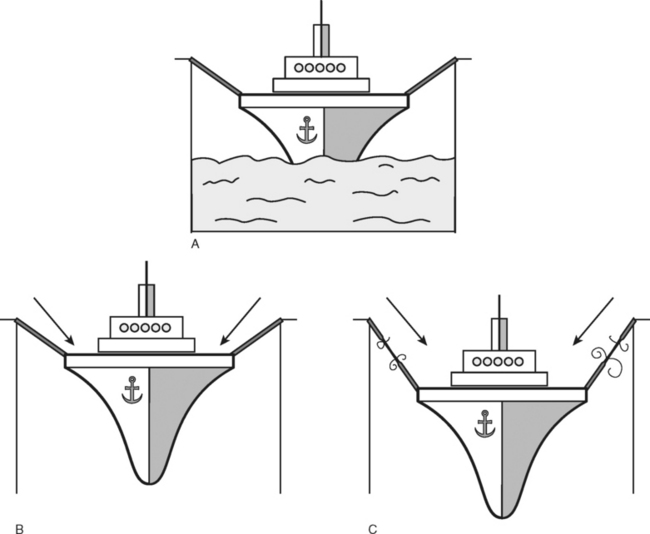
Support and suspension of the pelvic organs depends on a healthy innervated pelvic floor striated muscle, intact robust connective tissue, and their attachments to the bony frame of the pelvis. A useful analogy is that described by Peggy Norton, who suggested visualizing the relationships of these factors by considering a ship lying moored in dry dock. The ligaments are represented by the mooring ropes and the pelvic floor musculature by the water on which the ship sits. If the dry dock is emptied, then the influence of the supporting water disappears, leaving all of the tension applied to the mooring robes, which will inevitably weaken and fracture (Fig. 27-3).
The levator ani contains type I fibers that provide resting tone and type II (fast-twitch) fibers that maintain the urethral closure under stress and prevent stretching of the pelvic ligaments. Berglas and Rubin showed that in the nulliparous patients, the lower one third of the vagina is oriented more vertically and the upper two thirds deviate horizontally, thereby maintaining the vaginal axis in an almost horizontal position.29 This configuration is maintained by the posterior attachments of the cervix with the cardinal and uterosacral ligaments and by the anterior position of the urogenital hiatus. During stressful maneuvers, such as coughing or straining, the levator hiatus shortens anteriorly by the contraction of the pubococcygeus muscles. In the case if genital prolapse, when the levator ani support is lost, the vaginal axis becomes more vertical, the urogenital hiatus broadens, and fascial supports are strained.
EFFECT OF PREGNANCY AND CHILDBIRTH ON THE PELVIC FLOOR
An important pathophysiologic factor is the contribution of pregnancy and childbirth to the development of urinary incontinence in women.30,31 Epidemiologic studies have reported a prevalence of stress incontinence ranging from 23% to 67 % during pregnancy and 6% to 29% after childbirth. The fear of pelvic floor trauma has been used to justify an increase in the number of indiscriminate and often unnecessary cesarean deliveries.32
Our knowledge of pathophysiologic impacts of pregnancy and delivery on lower urinary tract function and the development of urinary incontinence remain scanty and often are somewhat confused.33 Future works needs to investigate the relative contribution provided by the individual components of pregnancy and delivery. The findings could significantly alter our understanding and treatment of stress incontinence.
It is a widely held view that stress incontinence is principally the consequence of delivery trauma to the pelvic floor. However, many studies have demonstrated that about 40% of nulliparous women experience occasional stress incontinence, which is a significant problem in 5% of them.34,35 The problem regularly worsens during the first pregnancy, and for the women developing stress incontinence in middle life, pregnancy rather than delivery seems to unmask the problem and worsen it.36 If incontinence emerges for the first time during pregnancy, it tends to resolve after the puerperium, but it may return in future pregnancies, progressively worsening and becoming a significant problem often many years after the pregnancies.
The prevalence of persistent, clinically important stress incontinence is significantly higher with greater multiparity than in nulliparous women, and it can be related to the number of pregnancies.37 Women who develop stress incontinence during pregnancy are more likely to experience incontinence later in their life. A study by Viktrup and colleagues38 of 278 women showed that 30% of them had stress urinary incontinence 5 years after delivery. In the group without stress urinary incontinence during pregnancy or after delivery, the incidence 5 years after delivery was only 19%. In the group developing stress urinary incontinence during the first pregnancy or puerperium with resolution of symptoms within 3 months after delivery, 42% of women had complained of recurrent incontinence 5 years later. In the latter group in which the symptoms of stress urinary incontinence did not resolve 3 months after delivery, the symptoms were still present in 92% 5 years later.38
Vaginal delivery has been recognized as being potentially traumatic to the pelvic floor. The first delivery may initiate injury to the continence mechanism as a consequence of direct damage to the pelvic floor muscles or nerves, or both, during the passage of the fetus. The perineal branch of the pudendal nerve courses in Alcock’s canal, lateral and anterior to the vagina, making it vulnerable to damage during childbirth.39 Additional deterioration of the urethral musculature and denervation can occur with aging, producing clinical disability many years after the initial trauma.40
The fetal head may dilate and overstretch the vaginal wall or avulse the cardinal and uterosacral ligaments, injuring connective support tissues. Moreover, the presenting part of the fetus during labor may constrict the pelvic structures, resulting in an ischemic injury. Compression of the fetal head against the subpubic urethra or paravaginal attachments may directly injure the urinary tract during labor and delivery. There are many mechanisms by which vaginal delivery can increase the risk of developing stress incontinence.41
The contribution of vaginal birth to producing denervation has been clarified by Allen’s electromyographic studies, which were performed before and after vaginal birth. Allen and colleagues42 found that most women have signs of neurologic damage after vaginal birth (but not after cesarean section), as confirmed by an increased motor unit potential, and increasing amounts of damage correlated well with greater evidence of stress urinary incontinence.
Multiparity, a long second stage of labor (>30 minutes), the use of the forceps, high birth weight (>4 kg), and a third-degree perineal tear are important risk factors for pudendal nerve damage.43 After spontaneous and instrumental deliveries, 21% and 34% of women complained of stress urinary incontinence and 5.5% and 4% reported fecal incontinence, respectively. Only 22% of patients incontinent during pregnancy continued to complain about it after delivery.44
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree