Fig. 19.1
Adenoma-like DALM with low-grade dysplasia, characterized by nuclear stratification and hyperchromasia
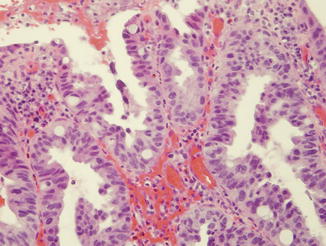
Fig. 19.2
High-grade dysplasia exhibits greater cytologic atypia, loss of nuclear polarity and architectural atypia
Endoscopic Classification and Terminology of Dysplasia in IBD
Historically, dysplasia in IBD has been categorized grossly (endoscopically) as either flat or elevated (DALM), the latter separated into adenoma-like and non-adenoma-like subcategories. In this categorization scheme, flat dysplasia was defined as dysplasia identified in random biopsies of colonic mucosa in which the area of dysplasia was endoscopically undetectable. More recently, particularly with the advent of advanced endoscopic techniques such as chromoendoscopy and confocal laser endomicroscopy, many lesions that were historically considered flat (endoscopically undetectable) can now be recognized endoscopically and, thus, targeted for biopsy. Thus, the term “flat” dysplasia is now considered somewhat misleading and often confusing. Furthermore, separation of elevated lesions (DALMs) into adenoma-like and non-adenoma-like suffers from interobserver variability. As a result, in March 2014, an international group of gastrointestinal specialists—including surgeons, gastroenterologists, and pathologists—convened in San Francisco to discuss, modify, and propose a revised categorization scheme of dysplasia in IBD that would be useful clinically and for future investigations: Surveillance of Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients: International Consensus Recommendations (SCENIC). The participants of this meeting agreed that the term “DALM” should be abandoned. The newly proposed endoscopic classification of dysplasia in IBD separates dysplasia into “visible” and “invisible” categories (by endoscopy). Visible dysplasia is subcategorized as polypoid or non-polypoid. Polyps may then be separated into those are pedunculated versus those that are sessile. Pedunculated lesions are those that are attached to the mucosa by a stalk, whereas sessile lesions do not have a stalk. However, in contrast, the entire base of the polyp is contiguous with the mucosa. Non-polypoid lesions (those previously considered flat) are now separated into those that are superficially elevated (lesions that protrude <0.25 mm above into the lumen, which is less than the closed cup on a forced biopsy forceps), flat (lesions without protrusion above the level of the mucosa), depressed (lesions with at least a portion depressed below the level of the mucosa), and ulcerated (lesions with ulceration and depressed fibrinous-appearing base). In contrast, invisible dysplasia is considered lesions that are identified on random (non-targeted) biopsies of colonic mucosa. The results of this meeting in San Francisco, and the classification system the participants proposed, have not yet been published at the time of the writing of this manuscript, so this system is considered tentative. Thus, for the purposes of this manuscript, the traditional endoscopic classification system of dysplasia (flat, elevated) is used.
Although surveillance strategies in IBD were originally developed based on the risk associated with “flat” dysplasia, several retrospective studies have revealed that most dysplasia identified in IBD patients is actually elevated. Blonski et al. [21] reported the endoscopic features of 58 sequentially identified dysplastic lesions in IBD surveillance endoscopies from a single institution. Fifty-one (87.9 %) of the biopsies were from visible lesions, 38 (66 %) of which were described as polyps, 12 (21 %) as ulceration/nodularity, and 1 (2 %) as a “circumferential lesion.” Rubin et al. [22] provided the endoscopic features of 75 neoplastic lesions identified in 1,339 sequential UC surveillance endoscopies. They reported that 46 (61 %) of the lesions were visible, including 23 (30 %) polyps, 22 (29 %) areas of “irregular mucosa” and 1 (1 %) stricture. Rutter et al. [23] reported similar findings in a retrospective review of 110 sequential neoplastic lesions identified in UC patients. Eighty-five (77 %) of the lesions were visible and 74 (67 %) were described as polyps. The high relative incidence of polypoid lesions in IBD emphasizes the importance of accurate endoscopic classification of visually identified dysplastic lesions in IBD.
Historically, raised dysplastic lesions have been referred to by the acronym DALM (Dysplasia Associated Lesion or Mass). As mentioned previously, use of this term is discouraged in favor of a new classification system proposed by SCENIC. DALMs have traditionally been subclassified as “adenoma-like” or “non-adenoma-like” based solely on their endoscopic features. Accurate endoscopic categorization of DALMs is critical since the management of adenoma-like and non-adenoma-like DALMs differs considerably. Adenoma-like DALMs resemble sporadic adenomas that occur in non-IBD patients. These lesions are typically well circumscribed with well demarcated borders and a smooth surface contour. In contrast, non-adenoma-like DALMs exhibit features not classically seen in sporadic adenomas. These include a broad plaque-like growth pattern, ulceration, stricture formation, multinodularity, irregular borders, and hemorrhage. Unfortunately there is some degree of interobserver variability in the endoscopic diagnosis of DALMs among practicing gastroenterologists [24]. For management purposes, endoscopically resectable DALMs can be considered “adenoma-like” and non-endoscopically resectable DALMs considered “non-adenoma-like.”
Adenoma-Like DALM Versus Sporadic Adenoma
Pathogenetically, adenoma-like-DALMs may represent either sporadic adenomas that have incidentally developed in IBD patients or polypoid dysplasia related to the patient’s underlying chronic colitis. Several studies have investigated clinical, morphologic, immunohistochemical and molecular features in attempts to distinguish “sporadic” adenomas from IBD-related polypoid dysplasia. Although some features, such as young patient age and longer duration of colitis, may favor IBD-related polypoid dysplasia over a sporadic adenoma [25], ultimately it is not usually possible to differentiate between these two entities based on routine pathologic analysis. One exception is that an adenoma-like-DALM arising in a region of the colon without current or prior involvement by colitis can generally be considered a sporadic adenoma. Dysplasia arising in IBD is believed to occur as a direct effect of inflammation. There is no evidence to suggest that mucosa uninvolved by colitis is at an increased risk of neoplasia. However, accurate information regarding the true extent of a patient’s disease is necessary before designating a segment of colon as uninvolved by prior colitis, since mucosa may normalize after treatment.
Torres et al. [26] compared the morphologic features of 89 adenoma-like DALMs in 59 IBD patients (51 with UC and 8 with CD) to sporadic adenomas. In this study, adenoma-like DALMs located within an area of colitis were designated probable IBD-associated polypoid dysplasia if flat dysplasia or adenocarcinoma was detected during a median follow-up period of 13 months. Adenoma-like DALMs located in segments of the colon uninvolved by colitis were designated sporadic adenomas. The mean duration of disease was longer in patients with IBD-associated polypoid dysplasia (11 versus 5 years). Morphologic evaluation revealed that lesions designated as IBD-associated polypoid dysplasia were more likely to have increased mononuclear lamina propria inflammation (60 % versus 16 %), tubulovillous/villous architecture (20 % versus 0 %), and mixture of normal and dysplastic crypts at the polyp surface (60 % versus 16 %). Although some differences in morphology were noted there is sufficient overlap between the groups to preclude distinguishing a sporadic adenoma from polypoid IBD-associated dysplasia in an individual case.
Adenoma-like DALMs have molecular features similar to those seen in sporadic adenomas. Fogut et al. [27] compared genetic alterations on chromosome 3p of adenoma-like (n = 18) and non-adenoma-like (n = 12) DALMs in UC patients and sporadic adenomas from non-IBD patients (n = 23). Loss of heterozygosity (LOH) of chromosome 3p markers D3S1766, D3S2409, and D3S2387 was detected in 70 %, 37 %, and 57 % of non-adenoma-like DALMs, respectively. In contrast, a low rate of LOH for these markers was seen in both sporadic adenomas (10.5 %, 7.1 %, and 0 %) and adenoma-like-DALMs (8.3 %, 11.7 %, and 15.3 %). In a similar study, Odze et al. [28] evaluated molecular features of adenoma-like (n = 12) and non-adenoma-like (n = 21) DALMs in UC patients and sporadic adenomas from non-IBD patients (n = 23). A high frequency of LOH for p16 (56 %) and 3p (50 %) was detected in non-adenoma-like DALMS. Both adenoma-like DALMs and sporadic adenomas exhibited a lower frequency of LOH for 3p (5 % and 28 %) and p16 (4 % and 5 %), respectively.
Walsh et al. [29] evaluated the immunophenotype of 38 adenoma-like DALMs in patients with UC and 13 sporadic adenomas from non-IBD patients as controls. Adenoma-like DALMs located outside areas of colitis in patients with no adenocarcinoma or flat dysplasia detected during follow-up were designated as sporadic adenomas. Adenoma-like DALMs located within areas of colitis that were associated with the development of flat dysplasia or adenocarcinoma at the same site within one year were designated as polypoid IBD-associated dysplasia. The frequency of p53 positivity was lower in sporadic adenomas from UC patients (5 %) and non-IBD control patients (15 %) than in polypoid IBD-associated dysplasia (29 %). In contrast, nuclear beta-Catenin positivity was higher in sporadic adenomas from UC patients (40 %) and non-IBD control patients (46 %) than in polypoid IBD-associated dysplasia (8 %).
Although some differences have been reported, there is significant overlap in the morphologic, immunohistochemical, and molecular features of sporadic adenomas and polypoid IBD-related dysplasia. However, as will be described later, the management strategy for an adenoma-like-DALM is the same regardless of whether it represents an incidental sporadic adenoma or polypoid IBD-related dysplasia.
Natural History and Treatment of DALMS
Early studies of DALMs reported a high rate of synchronous or metachronous invasive adenocarcinoma [30, 31]. However, it is now recognized that most initial reports were composed predominantly of lesions that would currently be designated as non-adenoma-like DALMs [32]. In the first description of DALMs in patients with UC by Blackstone et al. [31] in 1981, 7 of 12 (58 %) DALMs were associated with an invasive adenocarcinoma. These DALMs included “multiple sessile polyps” and “plaque-like” lesions. Endoscopic biopsies in many patients represented superficial sampling of an underlying invasive adenocarcinoma. The high risk of malignancy associated with these lesions emphasizes the importance of careful endoscopic characterization of polypoid lesions as adenoma-like or non-adenoma-like. The presence of a non-adenoma-like DALM (non-endoscopically resectable polypoid dysplasia) is an indication for colectomy due to the high risk of invasive carcinoma. However, as will be described later, more conservative management is appropriate for patients with an adenoma-like DALM.
Evidence supporting conservative management of adenoma-like DALMs emerged in the early 1990s with several reports of UC patients treated with polypectomy and continued endoscopic surveillance [33–35]. Subsequent larger studies with longer follow-up confirmed these initial observations. For example, Engelsgjerd et al. [36] evaluated outcomes of 24 UC patients with adenoma-like DALMs located within an area of colitis treated by polypectomy and surveillance compared to a control group of 49 non-IBD patients with sporadic adenomas. Eleven percent of the DALMs harbored high-grade dysplasia. In a subsequent publication, follow-up of this cohort was extended to a mean of 82 months for patients with an adenoma-like DALM within an area of colitis and 72 months for the control group [37]. Of the patients with an adenoma-like DALM, one (4 %) developed an invasive adenocarcinoma 7.5 years after polypectomy. Flat low-grade dysplasia was detected in a resection specimen from one patient (4 %). None of the other patients developed adenocarcinoma or dysplasia. Although the risk of developing adenocarcinoma or flat dysplasia during the follow-up period was low, subsequent adenoma-like DALM(s) were identified in 62.5 % of patients. However, this was not significantly different than the proportion of non-IBD control patients with an adenoma treated by polypectomy who developed subsequent adenomas.
Conservative management of adenoma-like DALMs in UC is also supported by a recent study by Kisiel et al. [38] evaluating 77 UC patients with adenoma-like DALMs inside (57 %) or outside (43 %) areas of colitis treated with polypectomy and endoscopy surveillance. During a median follow-up period of 20.1 months, flat low-grade dysplasia was indentified in four patients (5 %) and one patient (1.3 %) developed an invasive ileocecal valve adenocarcinoma. Twenty-eight patients (36 %) developed another adenoma-like DALM.
Goldstone et al. [39] conducted the largest study to date of adenoma-like DALMs in UC treated by polypectomy and endoscopic surveillance. Outcomes from 89 patients with adenoma-like DALMs were reported with a mean follow-up of 37.5 months. During the follow-up period 4 patients (4.5 %) developed adenocarcinoma. Subsequent high-grade dysplasia was indentified in 3 patients (3.4 %). However, the authors do not specify if the dysplasia was detected in flat biopsies or in additional adenoma-like DALMs.
It is important to emphasize that conservative management of adenoma-like DALMs requires that the polyp be completely removed with negative margins. Vieth et al. [40] compared outcomes of UC patients with adenoma-like DALMs completely removed by polypectomy (n = 87) or only biopsied (n = 60). Of the patients who underwent complete polypectomy, two (2.3 %) developed adenocarcinoma during a mean follow-up period of 53 months. In contrast, 10 patients (16.7 %) with adenoma-like DALMs that were only biopsied developed adenocarcinoma during a mean of follow-up of 87 months.
A recent a meta-analysis by Wanders et al. [41] evaluates 10 studies of DALMs treated by polypectomy in patients with UC. A total of 376 patients were included in the meta-analysis with an average follow-up of 54 months. Overall, 2.4 % of patients developed colorectal adenocarcinoma following polypectomy and surveillance. The pooled rate of adenocarcinoma was 5.3 cases per 1,000 patient years of follow-up. The low incidence of adenocarcinoma in this pooled analysis supports polypectomy and endoscopic surveillance as an appropriate management strategy for UC patients with an adenoma-like DALM. A treatment algorithm for DALMs is provided in Fig. 19.3. A summary of follow-up studies of adenoma-like DALMs treated with polypectomy and surveillance is shown in Table 19.1.
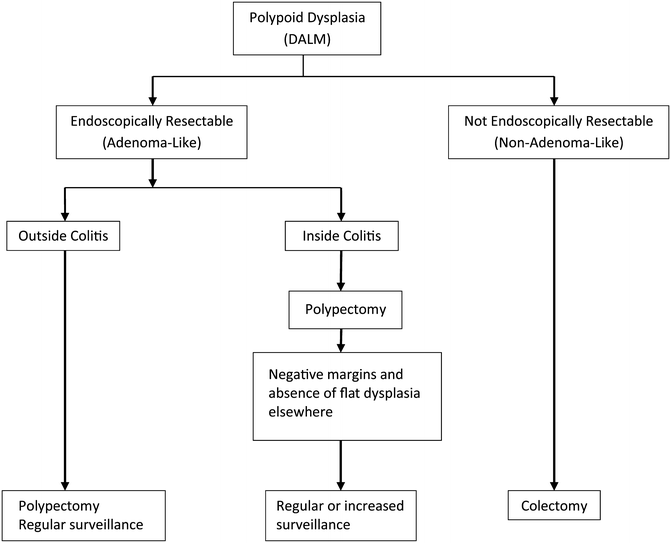

Fig. 19.3
Management scheme for polypoid dysplasia in IBD
Table 19.1
Studies of adenoma-like DALMs treated by polypectomy and surveillance
Authors | Year | IBD type | Number of cases | Follow-up | Subsequent carcinoma | Subsequent flat dysplasia |
|---|---|---|---|---|---|---|
Kisiel et al. | 2012 | UC | 77 | 20.1 months (median) | 1.3 % | 5.1 % |
Quinn et al. | 2012 | CD | 50 | 39 months (median) | 2.0 % | 2.0 % |
Goldstone et al. | 2011 | UC | 89 | 37.5 months (mean) | 4.5 % | 3.4 % |
Vieth et al. | 2006 | UC | 87 | 53 months (mean) | 2.3 % | 4.6 % |
Odze et al.a | 2004 | UC | 34 | 82.1 months (mean) | 2.9 % | 2.9 % |
Engelsgjerd et al. | 1999 | UC | 34 | 42 months (mean) | 0 % | 2.9 % |
Rubin et al. | 1999 | CD or UC | 48 | 49.2 months (mean) | 0 % | 0 % |
Dalms in Crohn’s Disease
Most studies of DALMs have been composed predominantly of patients with UC. However, early outcome studies by Rubin et al. [42] and Jess et al. [43] contained a small subset of patients with Crohn’s disease, suggesting that DALMs in CD may also be managed by polypectomy and endoscopic surveillance. In a recent paper, Quinn et al. [44] reported outcomes of 50 Crohn’s disease patients with adenoma-like DALMs treated by polypectomy over a median follow-up period of 39 months: 43 % of the DALMs occurred in areas of colitis and 57 % were located in areas without concurrent or prior involvement by colitis. High-grade dysplasia was present in 7 % of the polyps. During the follow-up period, one patient (2 %) developed invasive adenocarcinoma at a location distant from the polypectomy site. This patient was also noted to have flat dysplasia in her colectomy specimen. No other patients developed flat dysplasia or adenocarcinoma. However, 44 % of patients developed an additional adenoma-like DALM. The relatively high number of patients with CD that develop subsequent adenoma-like DALMs is similar to that reported in UC [37, 42]. The low incidence of subsequent adenocarcinoma and flat dysplasia reported by Quinn et al. [44] suggests that adenoma-like DALMs in CD can also be safely treated by polypectomy and continued endoscopic surveillance.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








