Pathology of Pancreatic Cancer
David S. Klimstra
N. Volkan Adsay
Although the most common neoplasm of the pancreas, infiltrating ductal adenocarcinoma (“pancreatic cancer”), constitutes more than 85% of primary pancreatic neoplasms, a wide array of other distinctive tumor types can arise in this organ (1). Most epithelial neoplasms of the pancreas exhibit differentiation along the lines of one of the three normal epithelial cell types, the ductal, acinar, or islet (endocrine) cells (1,2,3). The type of cellular differentiation is one of the major bases for pathological classification of pancreatic neoplasms (Table 27.1) because most entities display a specific cell phenotype as judged by their microscopic appearance or their immunohistochemical properties (3). Rarely, however, neoplasms exhibit differentiation along multiple lines, and there is a pancreatic neoplasm (solid pseudopapillary neoplasm [SPN]) for which the specific line of cellular differentiation is unknown (4). In addition to cellular differentiation, the gross configuration of the tumor (solid, cystic, intraductal, etc.) is used in the pathological subclassification. Of particular recent interest is a family of intraductal neoplasms of the pancreas that includes intraductal papillary mucinous neoplasms and other more recently described variants (5); these neoplasms represent a clinically detectable form of preinvasive neoplasia that may be cured by complete surgical resection in the majority of patients, provided they are detected before the development of invasive carcinoma. In addition, studies of intraductal papillary mucinous neoplasms provided evidence of more than one pathway for the development of invasive carcinoma in the pancreas (6,7). Since the early 2000s, more information has been accumulated regarding the clinical and pathological features of these and other less common pancreatic neoplasms, and the volume of data regarding the molecular genetics of pancreatic tumors has also grown exponentially. This chapter provides an overview of the pathological features of pancreatic neoplasms with an emphasis on diagnostic criteria, prognostic information, and natural history.
Infiltrating Ductal Adenocarcinoma
The most common neoplasm of the pancreas is infiltrating ductal adenocarcinoma. In the United States, approximately 32,000 new cases occur each year, representing the fourth leading cause of cancer death in both men and women (8). Despite recent advances in the surgical and medical treatment of ductal adenocarcinoma, the 5-year survival rate remains <5%. Even the minority of patients who present at a sufficiently early stage to undergo surgical resection have a 5-year survival of only 12% to 15% (9,10). It is particularly sobering to note that in follow-up of 5-year survivors, a significant proportion still succumb to pancreatic cancer following late recurrences (11). Because of the high rate of unresectability and the increasing use of neoadjuvant therapy prior to surgery for those with resectable disease, the initial pathological specimen from many patients consists of core biopsies and fine-needle aspiration specimens, either from the primary tumor or from a metastasis. The distinction of ductal adenocarcinoma from chronic pancreatitis, a common coincident finding in pancreatic cancer patients, can be particularly challenging on the basis of biopsy specimens, and it is this differential diagnosis that constitutes one of the more challenging aspects of pancreatic pathology.
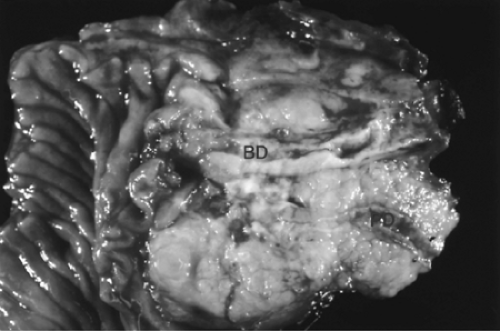
The gross and microscopic features of infiltrating ductal adenocarcinoma are usually quite characteristic (12,13,14,15,16). Approximately two-thirds of ductal adenocarcinomas arise in the head of the pancreas, with the remainder involving the body, tail, or entire gland (14,16). Multifocal invasive carcinoma is uncommon but is described. Most of the resectable ductal adenocarcinomas are relatively small (<3 cm), especially those involving the head of the gland (17,18); due to their highly aggressive nature, it is uncommon for ductal adenocarcinomas to attain large sizes in the absence of detectable distant metastases. The typical gross appearance is that of a remarkably firm, ill-defined solid mass that can merge imperceptibly with areas of fibrosing chronic pancreatitis (Fig. 27.1). Direct invasion into adjacent structures is typical, including the distal common bile duct, duodenum, ampulla of Vater or mesenteric vessels (for carcinomas of the pancreatic head), and the splenic vessels and spleen (for distally located carcinomas). In addition, extrapancreatic soft tissue invasion is frequent. Cystic change is described in ductal adenocarcinomas and may occur due to several mechanisms (19,20). In some cases, extensive central necrosis results in a degenerative cyst that can simulate the appearance of a pancreatic pseudocyst. In other cases, the carcinoma may obstruct a pancreatic duct resulting in a ductal retention cyst adjacent to the carcinoma. Finally, rare ductal adenocarcinomas contain massively dilated invasive glands that may be perceived grossly as microcystic structures (1).
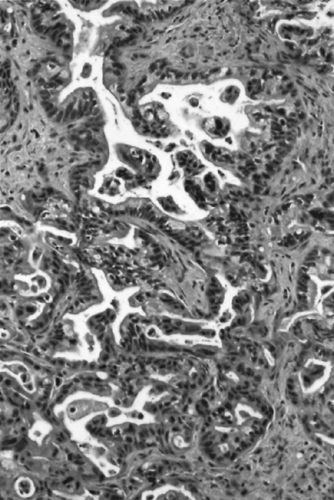
The microscopic appearance of conventional ductal adenocarcinomas is that of well-formed, round to angulated glands associated with a cellular, desmoplastic stromal response (Fig. 27.2) (13,14,21,22). This “tubular pattern” of infiltrating ductal adenocarcinoma is often associated with the other histologic variants, detailed later in this chapter. The neoplastic glands may be simple or branched and may be lined by a flat layer of epithelium or have architectural complexity such as cribriforming or micropapilla formation. It is also common to find small solid clusters of cells within the stroma, and individual cells may be present and difficult to distinguish from the activated myofibroblasts that constitute the desmoplastic response. The proportion of the tumor composed of well-formed glands, as
contrasted to solid nests and individual cells, is an important criterion for grading ductal adenocarcinomas (14,22,23), although it is common for both well-differentiated and poorly differentiated elements to coexist within an individual tumor. The neoplastic cells often contain relatively abundant cytoplasm rich in mucin. Although the nuclei of ductal adenocarcinomas may be superficially uniform, they are enlarged and usually vary in size, shape, and intracellular location from cell to cell within the individual glands. The finding of a 4:1 nuclear size variation is a helpful diagnostic feature. The highly invasive growth pattern of ductal adenocarcinoma is also evident microscopically. Even in well-differentiated examples, the individual glands extend into the uninvolved pancreatic parenchyma, and microscopic foci of carcinoma may be found well beyond the apparent gross limits of the tumor. Frequent perineural and vascular invasion is found as well as extension of carcinoma into peripancreatic adipose tissue (24). Invasion adjacent to muscular arteries is another helpful diagnostic feature because normal ducts are usually separated from the vasculature by lobules of acini. An additional characteristic feature of infiltrating ductal adenocarcinoma is the haphazard arrangement of the neoplastic glands, which contrasts with the lobular organization of ductules in atrophic chronic pancreatitis.
contrasted to solid nests and individual cells, is an important criterion for grading ductal adenocarcinomas (14,22,23), although it is common for both well-differentiated and poorly differentiated elements to coexist within an individual tumor. The neoplastic cells often contain relatively abundant cytoplasm rich in mucin. Although the nuclei of ductal adenocarcinomas may be superficially uniform, they are enlarged and usually vary in size, shape, and intracellular location from cell to cell within the individual glands. The finding of a 4:1 nuclear size variation is a helpful diagnostic feature. The highly invasive growth pattern of ductal adenocarcinoma is also evident microscopically. Even in well-differentiated examples, the individual glands extend into the uninvolved pancreatic parenchyma, and microscopic foci of carcinoma may be found well beyond the apparent gross limits of the tumor. Frequent perineural and vascular invasion is found as well as extension of carcinoma into peripancreatic adipose tissue (24). Invasion adjacent to muscular arteries is another helpful diagnostic feature because normal ducts are usually separated from the vasculature by lobules of acini. An additional characteristic feature of infiltrating ductal adenocarcinoma is the haphazard arrangement of the neoplastic glands, which contrasts with the lobular organization of ductules in atrophic chronic pancreatitis.
Table 27.1 Classification of Pancreatic Neoplasms | ||
|---|---|---|
|
As the infiltrating glands encounter other structures such as the bile duct, the small bowel mucosa, or even the native uninvolved pancreatic ducts, they commonly extend along the preexisting basement membranes, with the resulting growth patterns simulating a preinvasive neoplasm of the respective structures (1).
In addition to the conventional tubular pattern, ductal adenocarcinomas may focally or diffusely exhibit a variety of other histologic patterns such as adenosquamous, anaplastic, colloid, hepatoid, medullary, signet ring cell, and undifferentiated carcinomas. Most histologic variants of ductal adenocarcinoma carry the same prognosis as conventional tubular type adenocarcinoma. Some cases contain exceptionally well-differentiated glands with abundant microvesicular (foamy) cytoplasm and relatively uniform nuclei; this has been described as the foam cell pattern of ductal adenocarcinoma (Fig. 27.3)
(25). Approximately 4% of ductal adenocarcinomas have a substantial amount of the foam cell pattern. If due attention is not paid to subtle but distinctive morphologic features, foam cell carcinoma can be nearly indistinguishable from low-grade pancreatic intraepithelial neoplasia (PanIN), particularly in limited specimens such as cytologic preparations, frozen sections, or biopsy samples. The most characteristic feature is the abundant, pale, microvesicular cytoplasm with a peculiar condensation at the apical edge, which forms a brush borderlike zone that is not seen in benign mucinous glands (Fig. 27.3). Occasionally, invasive tubular adenocarcinomas have marked ectasia of the infiltrating neoplastic glands; some authors refer to this as “large duct-type” invasive carcinoma (26). This phenomenon is particularly pronounced in regions of the tumor infiltrating the overlying duodenal muscularis propria. The cytologic findings of the lining epithelium may be deceptively bland. Large duct-type adenocarcinomas should not be mislabeled as a mucinous cystadenocarcinoma, a neoplasm with a much better prognosis. Some ductal adenocarcinomas have a pattern similar to that of diffuse gastric carcinoma (27,28). Instead of the usual tubule formation, the cells form cords and are arranged individually, often with signet ring cells.
(25). Approximately 4% of ductal adenocarcinomas have a substantial amount of the foam cell pattern. If due attention is not paid to subtle but distinctive morphologic features, foam cell carcinoma can be nearly indistinguishable from low-grade pancreatic intraepithelial neoplasia (PanIN), particularly in limited specimens such as cytologic preparations, frozen sections, or biopsy samples. The most characteristic feature is the abundant, pale, microvesicular cytoplasm with a peculiar condensation at the apical edge, which forms a brush borderlike zone that is not seen in benign mucinous glands (Fig. 27.3). Occasionally, invasive tubular adenocarcinomas have marked ectasia of the infiltrating neoplastic glands; some authors refer to this as “large duct-type” invasive carcinoma (26). This phenomenon is particularly pronounced in regions of the tumor infiltrating the overlying duodenal muscularis propria. The cytologic findings of the lining epithelium may be deceptively bland. Large duct-type adenocarcinomas should not be mislabeled as a mucinous cystadenocarcinoma, a neoplasm with a much better prognosis. Some ductal adenocarcinomas have a pattern similar to that of diffuse gastric carcinoma (27,28). Instead of the usual tubule formation, the cells form cords and are arranged individually, often with signet ring cells.
Colloid (mucinous noncystic) carcinomas occur rarely in the pancreas (29); more than half of such cases arise in association with intraductal papillary mucinous neoplasms (IPMNs) (29,30). Colloid carcinomas consist of large extracellular lakes of mucin in which relatively minimal neoplastic epithelium is suspended (Fig. 27.4). These tumors are histologically similar to colloid carcinomas arising in the breast, colon, or other sites. Elements of conventional tubular-type ductal adenocarcinoma may occur together with the colloid carcinoma pattern, but for a carcinoma to qualify as colloid overall, at least 80% of the neoplasm must consist of extracellular mucin with suspended epithelium (29). When defined strictly, colloid carcinomas represent one of the more indolent types of invasive pancreatic adenocarcinoma (31,32,33). However, many cases occurring in association with IPMNs may have an invasive component limited to a few microscopic foci, raising the possibility that the favorable prognosis could be attributable to the early stage of many of the cases. A study of 17 colloid carcinomas limited to those cases having an invasive component >1 cm in size suggested otherwise, however, and the 5-year survival was 55% despite an average tumor size of 5.3 cm and regional lymph node metastases in eight cases (29). Thus, the favorable biology of colloid carcinoma seems to be an inherent property of this histologic variant. In addition to the morphologic differences from conventional ductal adenocarcinoma, colloid carcinoma commonly has an intestinal phenotype, with immunohistochemical expression of MUC-2 and CDX2 and negative labeling for MUC-1, in contrast to tubular-type adenocarcinomas having the opposite labeling pattern for these markers (23). The genetic alterations of colloid carcinomas are similar to those of conventional ductal adenocarcinomas, but occur at a lower rate. Only one-third of colloid carcinomas harbor mutations in codon 12 of the KRAS oncogene, and one-fourth have TP53 (p53) gene mutations (29). The expression of Smad4 (Dpc4) protein is normal in almost all cases (34).
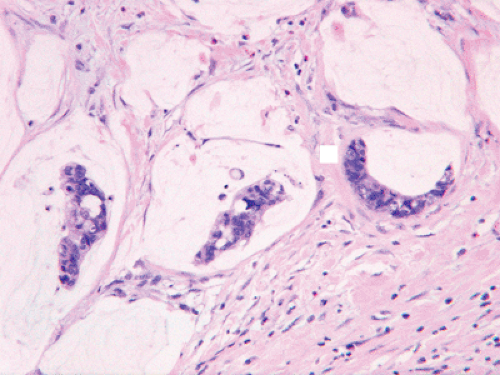 FIGURE 27.4. Colloid carcinoma. Strips of neoplastic glandular epithelium float in abundant extracellular pools of mucin (See also color Figure 27.4). |
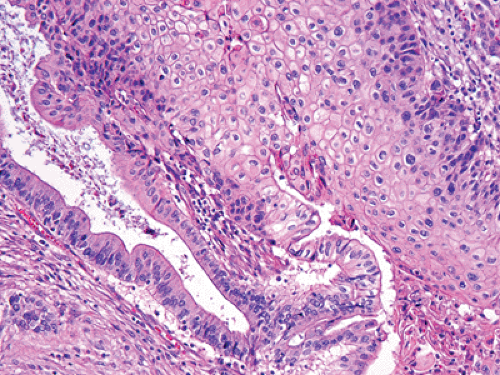 FIGURE 27.5. Adenosquamous carcinoma. Sheets of squamous cells (right) are juxtaposed to well-formed glands (left) (See also color Figure 27.5). |
Adenosquamous carcinomas constitute 3% to 4% of pancreatic carcinomas (35,36,37). In most of these lesions, a component of the tumor resembles conventional tubular adenocarcinoma. The squamous elements should arbitrarily constitute at least 30% of the neoplasm for it to qualify as an adenosquamous carcinoma (Fig. 27.5) (1). The clinical outcome of these tumors is as poor as that of ordinary (tubular) adenocarcinomas, if not worse.
Other tumors generally classified with ductal adenocarcinoma are the undifferentiated carcinomas. Several different types have been described in the pancreas. All may have associated components of tubular-type adenocarcinoma, or they may occur in pure form. Anaplastic giant cell carcinoma is a type of undifferentiated carcinoma in which the tumor cells are enormous (Fig. 27.6), with highly pleomorphic nuclei (38,39). The histologic pattern of anaplastic giant cell carcinoma of the pancreas resembles that of giant cell carcinomas of other organs such as the lung, adrenal, and liver (40). The cells are discohesive and often suspended in a sea of neutrophils, which may be present within the cytoplasm of some of the tumor cells (emperipolesis). Sarcomatoid carcinomas and carcinosarcomas are neoplasms having a spindle cell component, resembling a true sarcoma (41). In sarcomatoid carcinomas, the entire tumor is composed of spindle cells, which may retain epithelial differentiation at the immunohistochemical level. Carcinosarcomas are biphasic tumors that have an epithelial component and a separate sarcomatoid component, sometimes with heterologous stromal differentiation in the form of cartilage, bone, or striated muscle. Both components are believed to originate from epithelial precursors, however (42). All of these types of undifferentiated carcinoma are extremely rare in the pancreas, constituting <1% of its carcinomas, and prognostically are even more aggressive than conventional ductal adenocarcinomas.
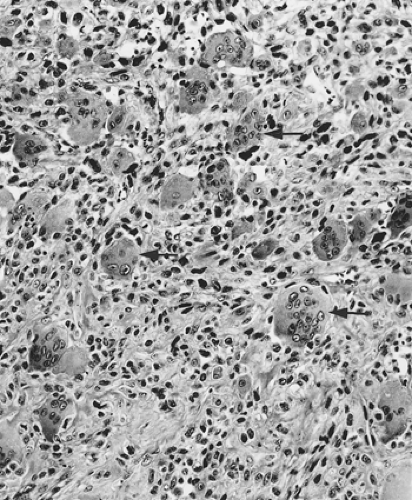
A separate and distinctive neoplasm is the undifferentiated carcinoma with osteoclastlike giant cells. This tumor consists of a neoplastic spindle to epithelioid cell component with admixed nonneoplastic osteoclastlike giant cells (Fig. 27.7) (43,44,45,46,47,48). Undifferentiated carcinomas with osteoclastlike giant cells are remarkably similar to osteoclast-containing tumors of many diverse organs, which suggests that some property of the neoplastic component may be responsible for the accumulation of intratumoral multinucleated giant histiocytes (44), which are formed from the fusion of circulating macrophages. Although the neoplastic components generally have a mesenchymal appearance and often fail to express evidence of epithelial differentiation by immunohistochemistry, the histogenesis of most cases is still believed to be epithelial, particularly in those cases associated with an adenocarcinomatous component. The finding of KRAS mutations in the tumor cells supports this hypothesis (49,50). In contrast to other types of undifferentiated carcinoma, undifferentiated carcinoma with osteoclastlike giant cells may have a more favorable prognosis, although recent studies suggested that most are quite aggressive (45).
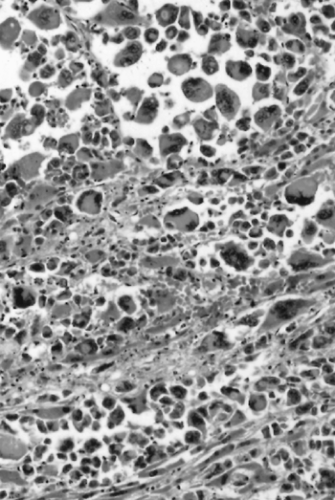
Another neoplasm, classified as a variant of ductal adenocarcinoma, is medullary carcinoma of the pancreas (51,52). The term medullary carcinoma is also applied to mammary and colorectal carcinomas that, despite a poorly differentiated histologic appearance, have a favorable clinical course. In the large bowel, medullary carcinomas are more frequent in patients with hereditary nonpolyposis colorectal cancer and commonly exhibit mutations in DNA-mismatch repair genes (53,54). Pancreatic medullary carcinomas are histologically similar to the mammary or colorectal counterparts (51,52). The tumors are poorly differentiated, lack gland formation, and have sheets of large cells with ill-defined borders suspended in a lymphocyte-rich stroma (Fig. 27.8). Patients more commonly have first-degree relatives with other extrapancreatic carcinomas than do patients with conventional pancreatic adenocarcinomas, but medullary carcinomas are not prone to occur more commonly in the setting of familial pancreas cancer. Pancreatic medullary carcinomas also more commonly have inactivation of DNA-mismatch repair genes than conventional ductal adenocarcinomas, and the KRAS oncogene is usually wild type. The first cases reported of pancreatic medullary carcinoma included some patients with a favorable outcome (51), but experience with additional cases has suggested that the prognosis is not as favorable as for medullary carcinomas of the breast or colorectum (52).
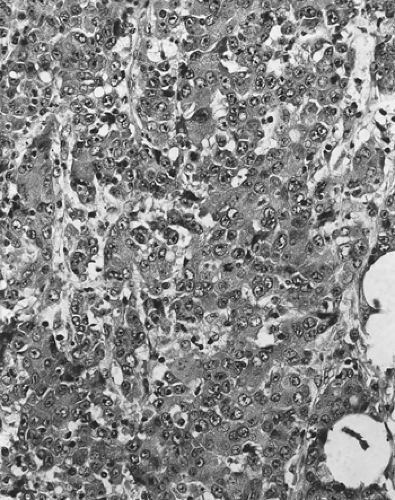 FIGURE 27.8. Medullary carcinoma. The tumor is poorly differentiated, with syncytially arranged cells lacking gland formation. A lymphocytic inflammatory infiltrate is present. |
Pancreatic Intraepithelial Neoplasia
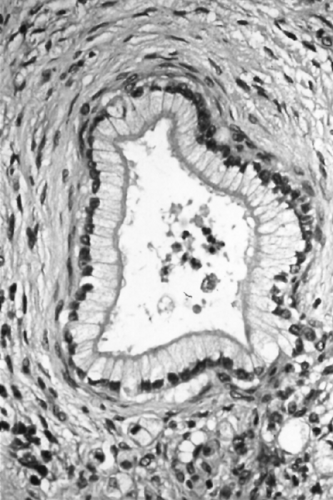
It is common to find a variety of ductal proliferative lesions associated with infiltrating ductal adenocarcinomas (Fig. 27.9) (55). The older terminology for these lesions included descriptive terms such as mucinous metaplasia (or mucous cell hypertrophy), papillary hyperplasia, atypical hyperplasia, and carcinoma in situ (21,56). Other investigators used grades of dysplasia. Recently, based on the recognition that these lesions formed a morphologic and genetic continuum of precursor lesions to infiltrating ductal adenocarcinoma (7,55,57,58,59,60,61,62), the unifying terminology of pancreatic intraepithelial neoplasia (PanIN) was adopted. Grades of PanIN include PanIN-1A, PanIN-1B, PanIN-2, and PanIN-3, based on the degree of cytoarchitectural atypia (14). PanIN1 is a common incidental finding that may occur in pancreata with or without a concomitant infiltrating ductal adenocarcinoma, whereas PanIN3 is much more closely associated with invasive carcinoma, rarely being detected in its absence. The progression of PanIN to invasive carcinoma is difficult to observe because PanINs are radiographically occult and are usually only identified in pancreatectomy specimens. However, a few rare cases of progression have been documented (57,58) that further support the precursor status of PanINs.
Most of the genetic abnormalities of infiltrating carcinoma are found at different stages in the PanIN sequence, including telomere shortening, and mutations in the KRAS, SMAD4, CDKN2A (p16), and p53 genes (33,63,64,65,66). What is most intriguing is that PanIN-1, the lesion historically regarded as simple mucinous metaplasia (tall columnar mucin-containing cells with basally located, uniform nuclei), has been found by several investigators to have KRAS mutations and telomere shortening (59,62,66), suggesting that it represents the earliest intraductal neoplastic alteration. This is an exciting observation, but one of uncertain practical value, for PanIN-1 is a common morphologic alteration, present in up to 45% of pancreata of older adults at autopsy (67). Nonetheless, the examination of early neoplastic lesions in the pancreas is one of the most interesting and promising avenues of study because early detection of pancreatic adenocarcinoma appears to represent the best chance for improvement in the nearly uniform lethality of this disease.
Intraductal Papillary Mucinous Neoplasms
Intraductal papillary mucinous neoplasms (IPMNs) are papillary tumors arising within the pancreatic ducts and often producing copious luminal mucin (14,31,68,69,70). These tumors
have been recognized as a distinct subset of pancreatic neoplasms for more than 20 years, but their prevalence appears to be increasing, perhaps due to the greater use of sensitive abdominal imaging. Alternative terms for IPMNs used predominantly in earlier reports include mucinous duct ectasia (71,72,73), papillary carcinoma, mucin-producing tumor, or villous adenoma of the pancreatic ducts (74,75).
have been recognized as a distinct subset of pancreatic neoplasms for more than 20 years, but their prevalence appears to be increasing, perhaps due to the greater use of sensitive abdominal imaging. Alternative terms for IPMNs used predominantly in earlier reports include mucinous duct ectasia (71,72,73), papillary carcinoma, mucin-producing tumor, or villous adenoma of the pancreatic ducts (74,75).
IPMNs occur in a patient population similar to that affected by ductal adenocarcinomas: adult patients, usually in the sixth to eighth decades of life (32,70,71). Men are affected more than women. The presenting symptoms are often vague and nonspecific, including abdominal pain, diarrhea, and other signs of exocrine insufficiency. Patients may have symptoms of chronic pancreatitis for many years before a diagnosis of IPMN is made. Biliary obstruction with jaundice is usually a late occurrence, often reflecting the development of invasive carcinoma.
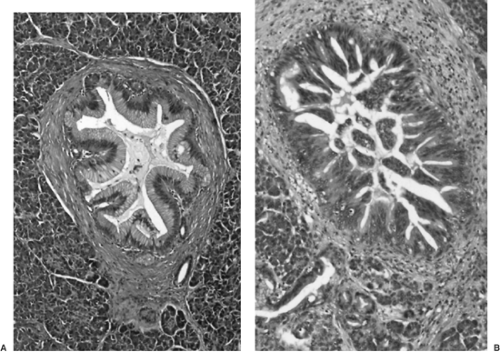
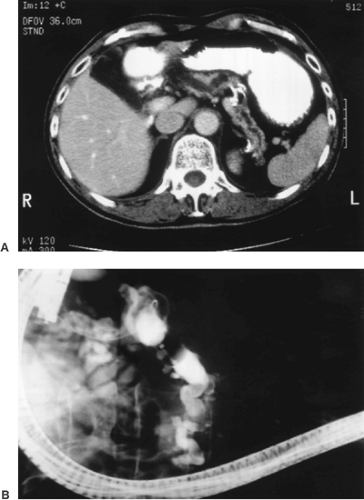
The majority of IPMNs (75%) involve predominantly the head of the pancreas; others are centered in the tail, and 5% to 10% diffusely involve the entire gland. Preoperative imaging may be very helpful in establishing a diagnosis of IPMN (69,70,76,77). Probably because of the excessive mucin production by the intraductal neoplasm, the pancreatic ducts involved by IPMNs commonly have significant dilatation, a finding that is usually apparent on computed tomography (CT) scanning, endoscopic retrograde pancreatography (ERCP), or magnetic resonance cholangiopancreatography (MRCP) (Fig. 27.10) (78,79). On cross-sectional imaging, the dilated ducts of IPMNs often appear as multiple separate cysts within the pancreas. This results from the fact that dilatated ducts become tortuous, so as to be cut multiple times in each plane of the CT scan. Mucinous cystic neoplasms (MCNs) instead appear as single multilocular cysts. IPMNs are separated into main duct and branch duct types, depending on whether the neoplasm is present within the major ducts or is limited to the peripheral secondary (branch) ducts (80,81). Branch duct–type IPMNs are particularly prone to resemble other cystic lesions of the pancreas because the connection to the ductal system can be difficult to recognize.
At endoscopy, the spillage of mucin from an engorged ampulla of Vater is a classic sign of IMPNs (73). Biopsy specimens taken from within the ampulla may disclose neoplastic papillary epithelium, often morphologically resembling a villous adenoma of the tubular gastrointestinal tract. Percutaneous biopsies or fine-needle aspiration procedures may also yield neoplastic epithelium and evidence of the luminal mucin accumulation (82,83). Correlating the results of biopsy procedures with the endoscopic and radiographic appearance of the lesion is important. Because the intraductal papillary proliferation may exhibit severe cytoarchitectural atypia in the absence of invasive carcinoma, the cytologic features of cells obtained from small biopsy or aspiration specimens may wrongly suggest the presence of invasive ductal adenocarcinoma if the intraductal location of the neoplastic proliferation is not apparent.
Pathologically, IPMNs vary in their gross and microscopic appearances (31,32,70,84). Depending on the extent of the tumor, diffuse ductal dilatation or localized cystic transformation of smaller ducts may be present. Some examples exhibit minimal gross evidence of papilla formation, appearing only as dilated, mucin-filled ducts (Fig. 27.11). Other cases have abundant intraductal papillae, with a velvety lining of tan papillations covering the entire luminal surface. The extent of ductal dilatation may be impressive, with some IPMNs having ducts larger than 5 cm in diameter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree