Fig. 12.1
Features of NAFLD. (a) Zone 3 distribution of steatosis in a case without fibrosis or features of steatohepatitis. Portal areas are indicated with arrowheads. (b) Zone 3 injury in a case of steatohepatitis. Steatosis, lobular inflammation, ballooning, and perisinusoidal fibrosis are all present. (c) Steatosis in NAFLD is generally macrovesicular, with a mix of small and large vacuoles. (d) Patches of microvesicular steatosis may be seen, particularly in severe cases of steatohepatitis. (e) Portal inflammation in NAFLD is generally mild or only focally of moderate severity. (f) Masson stain showing perisinusoidal fibrosis between the hepatocytes
Although steatosis may be present in a nearly pure form, as the disease progresses to steatohepatitis, other findings, particularly fibrosis, can disrupt the appearance of the steatosis, making the zonal distribution unclear [9]. Steatotic hepatocytes may have a mix of smaller and larger vacuoles and patches of steatosis may be distributed irregularly. As cirrhosis develops, the amount of steatosis decreases and can actually fall below the 5 % threshold, making it difficult to recognize that the disease resulted from NAFLD and NASH. Although many such cases were categorized as cryptogenic cirrhosis in the past, careful examination of the biopsy will usually reveal features of steatohepatitis, particularly ballooned hepatocytes and Mallory–Denk bodies [10, 11]. NASH may recur after transplantation [12].
True microvesicular steatosis, in which hepatocytes take on a foamy appearance with innumerable tiny vacuoles, may be seen sometimes in NAFLD, particularly when steatohepatitis is present (Fig. 12.1). Hepatocytes with this change may be found singly or in small patches. Another steatotic change, in which hepatocytes may show multiple vacuoles of variable size, is a common finding and should not be mistaken for microvesicular steatosis. Microvesicular steatosis tends to be found in more severe cases of steatohepatitis, but its clinical significance as an independent finding is still unclear [13].
Inflammation
The inflammation in NAFLD and NASH is conventionally divided into parenchymal inflammation and portal inflammation. In general, the severity of inflammation, particularly portal inflammation, increases as the disease progresses from steatosis to steatohepatitis, but there is enough variability that the severity of inflammation alone is not helpful in distinguishing steatohepatitis from steatosis alone.
The parenchymal inflammation consists of small foci of lymphocytes and macrophages, often appearing to infiltrate into liver cell plates (Fig. 12.1). These inflammatory foci are very similar to those seen in other chronic liver diseases. When the foci of inflammation are composed mainly of macrophages, they are called microgranulomas. Microgranulomas are a very common finding in NAFLD and sometimes may surround a lipid vacuole, presumably from a hepatocyte that has been destroyed. In this configuration, they resemble the “crown-like structures” described in adipose tissue of humans and animals with metabolic syndrome [14]. Apoptotic hepatocytes (acidophil bodies) may be seen associated with parenchymal inflammation or separate from inflammatory foci. They are seen more frequently as the disease severity increases [15, 16]. Foci of neutrophils are not seen as often in NAFLD as in ALD; however, when present, they are seen close to or surrounding balloon cells and Mallory–Denk bodies.
The portal inflammation in NAFLD and NASH is typically milder than seen in other chronic liver diseases. Most cases will show at least a few portal areas with a sparse infiltrate of lymphocytes and macrophages. As the fibrosis progresses, the number of portal areas with inflammation and the degree of inflammation within individual portal areas increases [17] (Fig. 12.1). This can cause confusion with chronic hepatitis in cases where the steatosis is mild and the balloon cells are not apparent. Plasma cells and eosinophils are rare when present and do not dominate the infiltrate. Interface hepatitis is sometimes seen, particularly when periportal fibrosis is present, but it is mild in comparison to the average case of chronic hepatitis, with focal involvement of a minority of portal areas.
A few studies have evaluated the character of the inflammation in NAFLD using immunohistochemical techniques. Lefkowitch et al. used anti-CD68 to study the distribution of macrophages in cases of NASH [18]. They found that clusters of hypertrophied macrophages seemed to be present more often in zone 3 and tended to be close to ballooned hepatocytes. In NAFLD cases without features of NASH, the aggregates of macrophages were not as prominent. Lymphocytic inflammation in the parenchyma was composed of nearly equal numbers of CD4-(+) and CD8-(+) T lymphocytes with very few B lymphocytes. With respect to the portal inflammation, Gadd et al. noted that the infiltrate was dominated by macrophages and CD8-(+) T cells [19]. Using data from paired biopsies, they suggested that increased numbers of portal macrophages may be present in cases of steatosis that progressed to steatohepatitis.
Ballooning
Ballooning hepatocellular injury is the characteristic cellular lesion of steatohepatitis [7] and current expert consensus suggests that without evidence of ballooning injury, a definite diagnosis of steatohepatitis cannot be made [2]. Because no noninvasive test can accurately detect the presence of balloon cells, a biopsy is required to distinguish NASH from cases with steatosis alone. Balloon cells are typically larger than normal hepatocytes, but they may not be larger than a hepatocyte containing a large fat vacuole (Fig. 12.2). The cytoplasm is irregularly clumped and stranded, leaving irregular translucent or optically clear spaces in between. Small fat vacuoles can sometimes be seen but should not dominate the cytoplasm. Ballooned cells are found most often in acinar zone 3 adjacent to strands of perisinusoidal fibrosis. When the ballooning injury is severe, the balloon cells may be large enough to recognize from low magnification, but it should be recognized that there is wide variation in the size and numbers of balloon cells between individual cases of steatohepatitis. In any case of fatty liver disease with characteristic fibrosis (described below) or clear zone 3-centered injury with steatosis and inflammation, a diligent search should be made for balloon cells. Examination of multiple sections may be necessary in order to find diagnostic cells.
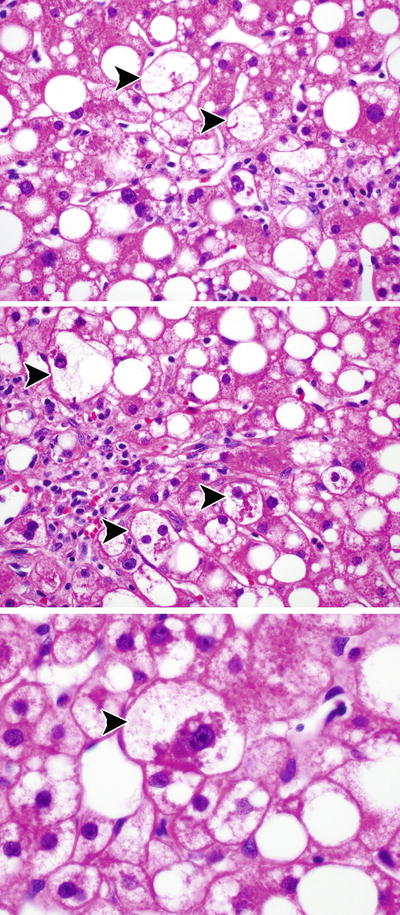

Fig. 12.2
Ballooned hepatocytes. The panels show examples of ballooned hepatocytes in NASH (arrowheads). Some contain Mallory–Denk bodies
Mallory–Denk bodies have long been recognized as a distinctive feature of ALD and were important in the recognition of NASH as a distinct form of liver disease in nonalcoholics. Mallory–Denk bodies are most easily identified when they appear within ballooned hepatocytes (Fig. 12.2). In such cases, they appear as irregular, densely eosinophilic, ropey cytoplasmic inclusions. Often they are closely associated with the nucleus either as an irregular inclusion to one side or surrounding the nucleus like a collar. Mallory–Denk bodies are composed of a large complex of hyperphosphorylated and misfolded filaments of keratins 8 and 18 [20]. Immunohistochemical staining for ubiquitin, p62, and keratin 8 and 18 can help to identify Mallory–Denk bodies, and these stains will also identify Mallory–Denk bodies in non-ballooned hepatocytes. Recent work has promoted the use of double immunostains for ubiquitin and keratin 8 and 18 to both help identify Mallory–Denk bodies and ballooned hepatocytes [21, 22]. In this stain, the Mallory–Denk bodies are double stained and the cytoplasm of ballooned cells will be negative. Since the surrounding normal and steatotic hepatocytes will stain positively with keratin 8 and 18, this double stain can help pathologists to be confident in their identification of balloon cells.
Fibrosis
Like most chronic liver diseases, NAFLD carries a risk to develop fibrosis and cirrhosis. Patients with steatohepatitis, rather than those with just steatosis, are considered to be at risk for cirrhosis and in fact most cases diagnosed as steatohepatitis will have some degree of fibrosis already present. Unlike chronic viral hepatitis and chronic cholestatic diseases, the fibrosis in steatohepatitis begins in zone 3 rather than around portal areas. This fibrosis takes the form of collagen deposition in the perisinusoidal space between endothelial cells and hepatocytes (Fig. 12.1, panel f). This leads to a network of fibrosis that surrounds and isolates individual hepatocytes extending out from the terminal hepatic venule. The earliest stages require the use of special connective tissue stains in order to visualize the fibrosis, but as the fibrotic network thickens and hepatocytes are lost, the fibrosis can be seen on routine stains. Periportal fibrosis develops next in most cases, with periportal hepatocytes trapped by collagen. Ductular reaction may be seen in these cases, particularly if keratin 7 or 19 stains are employed to highlight ductular epithelial cells. Bridging fibrosis follows, with fibrotic connections along zone 3 from portal areas to adjacent terminal hepatic venules. Bridging fibrosis between terminal hepatic venules can be observed, but pure portal–portal bridging fibrosis is unusual in adults with steatohepatitis. Cirrhosis that develops from steatohepatitis can resemble cirrhosis from other forms of chronic liver disease, particularly chronic viral or autoimmune hepatitis. Before NASH was recognized as a significant liver disease leading to cirrhosis, a significant minority of patients presenting to transplant centers were classified as having cryptogenic cirrhosis [11]. Studies of this patient population showed that many of these patients had risk factors for NAFLD and NASH and careful examination of explants revealed residual ballooning and Mallory–Denk bodies even though the degree of steatosis was minimal. Studies of patients with steatohepatitis documented prior to transplant confirmed this observation, suggesting that most patients undergoing transplant for cryptogenic cirrhosis actually had cirrhosis related to NASH [10].
A diagnostic dilemma arises when the biopsy shows steatosis, inflammation, and a characteristic fibrosis pattern but lacks definitive ballooning injury. As noted above, this should prompt a careful search for balloon cells, with the use of immunohistochemical studies for Mallory–Denk bodies if available. However, as with any histological lesion in the liver, the observation of ballooning injury may be subject to sampling adequacy and to observational variability [23]. Since ballooning is a required element for the diagnosis of definite steatohepatitis, one solution is to classify these cases descriptively as fatty liver disease with steatosis, inflammation (if present), and fibrosis but without diagnostic changes of steatohepatitis. Another approach is to classify these cases as having borderline changes of steatohepatitis and there is evidence to suggest that cases classified in this manner have clinical characteristics that fall between those of steatosis alone and definite steatohepatitis [1, 24]. Either approach is valid as long as there is clear communication between the pathologist and clinician and the biopsy is adequately described in the report.
Other Findings
There are a variety of other findings that may be seen in biopsies from patients with NAFLD. These include megamitochondria, cytoplasmic glycogenosis, glycogen nuclei, lipogranulomas, and iron. Of these, glycogenosis requires some attention because it is common and glycogenotic hepatocytes can mimic balloon cells or make them harder to identify. In glycogenosis, the hepatocytes are enlarged and the cytoplasm has a pale, amphiphilic appearance with delicate eosinophilic stranding. Most of the hepatocytes can be affected, resulting in an appearance similar to glycogenic hepatopathy. Glycogenic hepatopathy is an acute form of glycogenosis associated with type I diabetes and high aminotransferase levels that resolves once glucose levels are controlled [25]. The glycogenosis of NAFLD does not carry the same clinical significance although there is a weak association with type II diabetes [26]. Megamitochondria can be seen in NAFLD as in ALD. They are ovoid eosinophilic, periodic acid–Schiff reaction-negative, cytoplasmic inclusions [13, 27]. Their clinical significance in NAFLD is unclear. Glycogen nuclei are a common finding in NAFLD and NASH and they have been associated with diabetes. They do not have any clinical or diagnostic significance. Lipogranulomas may be seen in NAFLD as in other chronic liver diseases, but they are mainly associated with dietary mineral oil and are seen more frequently in older patients [28]. Because they can be located near terminal hepatic venules and associated with fibrosis, they can cause confusion in early-stage disease. The fibrosis associated with lipogranulomas should be discounted when assessing fibrosis in NAFLD.
Iron is often an incidental finding in biopsies performed to evaluate NAFLD. Because iron may act as a cofactor in hepatic disease progression, Nelson et al. examined the relationship of iron deposition and histological severity in NAFLD in a large patient cohort [29]. They found an association between iron accumulation in Kupffer cells and the severity of inflammation, ballooning, and fibrosis. Biopsies showing only hepatocellular iron had less injury than those with no stainable iron. The pathophysiology behind these differences is still unclear, although the authors speculated that there may be differences in hepcidin signaling that could lead to iron deposition in different cells.
Histopathological Features of NAFLD in Children
Children, particularly adolescents, may develop the same histological spectrum of NAFLD as adults, although the incidence of cirrhosis is less and the disease tends to be milder overall. Preadolescent children also have a form of fibrosing fatty liver disease with a different histological appearance than NAFLD in adults and adolescents [30]. This type of NAFLD was originally called type 2 steatohepatitis , but the NASH Clinical Research Network uses the term “zone 1 borderline pattern” because most cases lack typical balloon cells and so do not meet criteria for steatohepatitis [1]. This latter term also highlights the characteristic histological change—zone 1 predominant injury. Cases show the most severe macrovesicular steatosis in periportal hepatocytes, with decreasing amounts of fat as the terminal hepatic venules are approached (Fig. 12.3). The steatosis may also be pan-acinar, but the terminal hepatic venule region will be spared in terms of inflammation and fibrosis. The portal areas contain a mild, lymphocytic, and histiocytic infiltrate that is often associated with periportal fibrosis. Bridging fibrosis involves adjacent portal areas and the terminal hepatic venules are uninvolved. Connective tissue stains can be very helpful in bringing out the zone 1 injury pattern by highlighting early septum formation. Balloon cells are rarely found and are not as well formed as those seen in adult-type steatohepatitis. Mallory–Denk bodies are not seen, and their presence should suggest the diagnosis of steatohepatitis rather than the zone 1 pattern. This pattern is common among children with fatty liver disease, comprising 28 % of a cohort of US children between age 6 and 17 [31]. Among adults, blinded review finds only 1 % of cases to show the zone 1 injury pattern [24, 32]. Patton et al. examined the clinical characteristics of children with the borderline zone 1 pattern in comparison to children with definite steatohepatitis [31]. The zone 1 group were younger (mean age 11 vs. 13), less physically mature (Tanner stage 1.8 vs. 2.7), and more often of Hispanic ethnicity (69 % vs. 46 %) and had lower fasting insulin levels (23 vs. 36 mg/dL) with the same fasting glucose levels. The natural history of this form of fatty liver disease is unclear. The fact that the zone 1 pattern is not seen as often in adolescents suggests that either the disease shifts to zone 3 or resolves without progression.
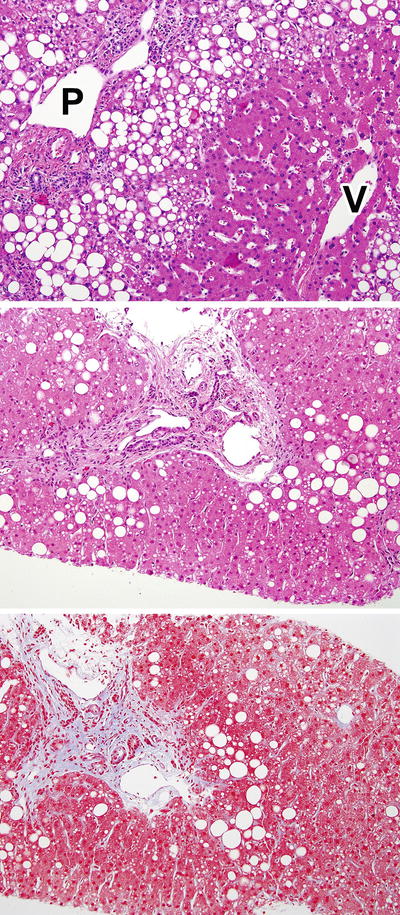

Fig. 12.3
Zone 1 borderline pattern. These panels illustrate the zone 1 pattern of injury seen most often in young children. In the top panel, there is moderate steatosis that hugs the edge of the portal area (P), while the terminal hepatic venule (V) shows no steatosis or injury. The bottom panels show H&E and Masson trichrome stained parallel sections with mild zone 1 steatosis associated with periportal fibrosis
NAFLD in Patients with Lipodystrophy
Lipodystrophies are a collection of disordered characterized by loss of adipose tissue (particularly from the subcutaneous compartment). The loss may be partial or generalized and both genetic and acquired forms are described [33]. The acquired forms may be related to drug injury, particularly with certain antiretroviral medications [34], or idiopathic, with a presumed autoimmune etiology. Patients have insulin resistance that is frequently severe, low adiponectin and leptin levels, and severe hypertriglyceridemia with low levels of high density lipoproteins. Most have NASH, probably related to their insulin resistance [35]. A recently published study described the liver biopsy findings in 50 patients (including both adults and children) with various forms of lipodystrophy [36]. Within this group, 82 % had definite or borderline steatohepatitis and 42 % had advanced fibrosis (bridging fibrosis or worse). The most advanced cases (and all cases of cirrhosis) were seen in patients with acquired generalized lipodystrophy and in patients with congenital generalized lipodystrophy who had a mutation in the seipin gene. In other respects, the character of the NAFLD in lipodystrophy was similar to that seen in patients with more common risk factors. Although fatty liver disease was the most common finding, four patients had histological and clinical evidence of autoimmune hepatitis. The cohort was treated with metreleptin in an open-label study since leptin therapy is known to ameliorate the insulin resistance in patients with lipodystrophy [37]. Follow-up biopsies performed in 27 patients showed reduction in steatosis, lobular inflammation, ballooning, and some resolution of steatohepatitis. Fibrosis remained unchanged in this population.
Steatosis and Steatohepatitis in Patients with Other Chronic Liver Diseases
Because steatosis is a common finding in liver biopsies, it is not unusual to find steatosis and more specific features of steatohepatitis in patients with other liver diseases. Steatosis and steatohepatitis may have diverse etiologies when found as a second disease process. Risk factors for ALD and NAFLD may be present, either alone or together. The disease process itself may increase the likelihood of steatosis for other reasons. The well-known association of infection with genotype 3 of hepatitis C and steatosis is probably the best example [8, 38]. Patients may be on medications such as methotrexate or tamoxifen that are associated with fatty liver disease [39]. Drugs may also cause weight gain [34, 40, 41] or lipodystrophy [42, 43], leading to steatosis by a secondary mechanism.
In one large single-center study, steatohepatitis was found as a complication in 5.5 % of cases of chronic hepatitis C and 4 % of other liver biopsies performed for other liver diseases [44]. Among the hepatitis C cases, 27 % had alcohol as a risk factor, but 60 % of these also had obesity and 25 % had diabetes as concurrent risk factors. Only 7 % were infected with genotype 3. Criteria for diagnosing concurrent steatohepatitis in the presence of other liver diseases should be strict, with unequivocal ballooning injury (Fig. 12.4). It is very helpful to find Mallory–Denk bodies and perisinusoidal fibrosis along with the balloon cells. Identification of concurrent steatohepatitis should prompt a search for the etiological risk factors and should be taken into account when using biochemical serum measurements to assess response to therapy of the primary disease.
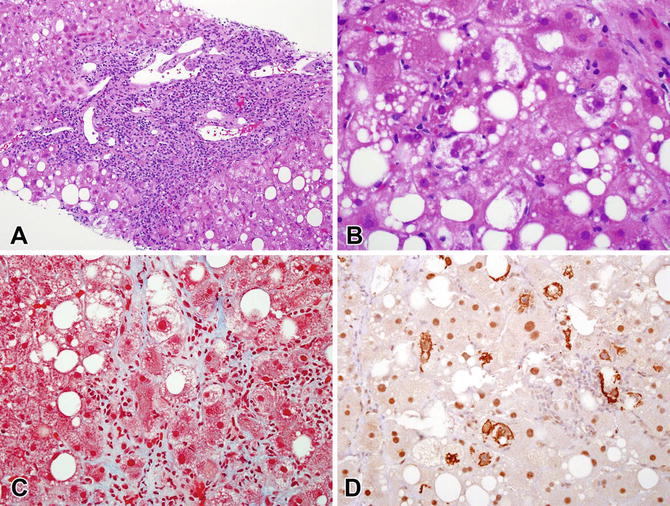

Fig. 12.4
Steatohepatitis in a case of chronic hepatitis C . (a) There is dense portal inflammation with interface hepatitis consistent with hepatitis C infection. Steatosis is present. (b) In zone 3, ballooning injury with Mallory–Denk bodies is seen. (c) The Masson trichrome stain showed characteristic perisinusoidal fibrosis in zone 3. (d) A ubiquitin immunostain highlights the many Mallory–Denk bodies that were present
Although excessive alcohol consumption is consistently identified as a risk factor for disease progression in chronic liver disease from other causes, evidence for a similar effect of NAFLD on disease progression has been more difficult to define. Most of the published studies have focused on chronic hepatitis C and have been limited to examining the relationship of steatosis, rather than steatohepatitis, on fibrosis progression. In cross-sectional studies, steatosis (mainly related to metabolic syndrome) has been associated with the stage of fibrosis [45–47]. The I148M polymorphism of the patatin-like phospholipase domain-containing 3 (PNPLA3) gene, which has been highly linked to NAFLD and NASH, has also been linked to increased cirrhosis prevalence in cross-sectional studies of hepatitis C [48]. A longitudinal study of chronic hepatitis C using paired biopsies failed to find an association between steatosis and progression of fibrosis despite confirmation of an association in cross-sectional analysis [49]. An association between fibrosis and steatosis has been reported in primary biliary cirrhosis [50] but not chronic hepatitis B [51, 52]. The potential relationship between NASH and fibrosis in other chronic liver diseases has not been studied extensively, possibly because of the much lower prevalence of NASH than steatosis.
Natural History of NAFLD in Biopsy Studies
Biopsy remains the reference tool for assessing the histopathological patterns and natural history of NAFLD since noninvasive markers have neither been fully validated in this context nor do they provide information on the whole histologic spectrum of NAFLD. However, as for any chronic liver disease, the liver biopsy has inherent limitations. Among them, sampling error is a significant concern. Indeed, the liver biopsy samples only a very tiny part of the whole organ and there is a risk that this part might not be representative of fibrosis and other lesions due to heterogeneity in disease distribution. In the context of NAFLD and in morbid obesity, several studies have underlined this risk although the magnitude of discrepancies is variable according to histological features and across the different studies [23, 53–56]. As in other chronic liver diseases, the risk of sampling error is decreased with increasing length of the liver biopsy. Except in the diagnosis of cirrhosis, for which micro-fragments may be sufficient, a 25 mm long biopsy is considered an optimal specimen for accurate evaluation, though 15 mm is considered sufficient in most studies [57]. The caliber of the biopsy needle is also important for getting an adequate biopsy of liver for evaluation. A 16 gauge needle is considered to be appropriate [58]. Another potential limitation is related to the discordance between pathologists in biopsy interpretation (interobserver variation), although it is less important when biopsy assessment is done by specialized liver pathologists [59].
The natural history of NAFLD is an active area of investigation. The broad outlines are clear—that NAFLD and particularly NASH are chronic liver diseases that may progress to cirrhosis. The details remain to be fully characterized. What patients are at greatest risk for fibrosis progression over the short and over the long term? What determines whether a patient with steatosis alone develops NASH or fibrosis? Does disease activity (and/or fibrosis) fluctuate with time, and if so, what clinical factors drive the improvement or worsening of disease? Understanding the short-term behavior of this disease has bearing on deciding which patients are most likely to benefit from a potential therapy, since most clinical trials test efficacy over a year or two. Given the metabolic nature of NAFLD, the treatment paradigm will ultimately be more like that of hypertension and diabetes than an infection like chronic hepatitis C.
Although some of the long-term issues can be examined by retrospective cohort studies with hard end points (death, transplantation, cancer), information useful for patient management is better derived from longitudinal studies where pairs of biopsies can be examined. A number of paired biopsy studies have been published in NAFLD, almost all of them in adult populations. These studies fall into two groups. One group consists of cohorts of patients collected retrospectively or prospectively and the outcome examined is fibrosis progression. The other group consists of placebo-treated patients in clinical trials. In this latter group, the outcome is usually fibrosis regression or other improvements in histological findings. Longitudinal cohorts typically have longer time intervals between the biopsies, on the order of 3–6 years, while placebo groups have a time between biopsies limited by the treatment period.
In one of the larger cohort studies published to date, Sorrentino et al. looked at paired biopsies in 132 patients [60]. With a mean time between biopsies of 6.4 years, they observed fibrosis progression in 45 and regression in 11. In their study, which was focused on the evaluation of fibronectin staining in baseline biopsies, they found that the degree of fibronectin staining, the presence of hypertension, and a HOMA-IR score of >10 were associated with increased risk of fibrosis progression. Although early cohort studies suggested that patients with steatosis alone or steatosis with mild inflammation were at little or no risk to progress, Pais et al. recently reported data on a cohort of 25 patients with NAFLD (but not NASH), 16 of whom developed steatohepatitis over a mean follow-up of 3.7 years [61]. Six of these patients developed bridging fibrosis. These data suggest that assumptions about benign clinical course cannot be made in patients who only have steatosis and inflammation on biopsy although differences in progression rates are likely to exist between patients with steatohepatitis and those with only steatosis. The NASH Clinical Research Network also recently presented data on their longitudinal cohort of 375 adults with paired biopsies followed for a median time of 4 years. In this group, 128 (36 %) showed fibrosis progression and 106 (28 %) showed fibrosis regression [62]. Although baseline clinical data was of no value in predicting which patients would progress or regress, changes in BMI, aminotransferases, and alkaline phosphatase were all clearly associated with changes in fibrosis.
The PIVENS clinical trial of vitamin E and pioglitazone is one of the largest placebo-controlled studies to publish information on its placebo group [63]. Out of 72 placebo-treated patients, 22 had apparent regression of fibrosis. While some “improvement” in fibrosis can be attributed to observational variability, these patients also improved clinically, with loss of weight in some and decreased aminotransferases overall. Others [64–66] have also reported improved histological findings in placebo groups. These findings, along with the findings of fibrosis progression and regression in longitudinal cohorts, suggest that NAFLD may have a fluctuating natural history depending on patient-initiated lifestyle modifications and other factors that may be difficult to model or anticipate.
Staging and Grading in NAFLD
Because the liver biopsy remains important in the evaluation of the natural history and in clinical trial outcomes, it is important to have structured methods to assess histological change in the liver biopsy. Like chronic hepatitis, there are several staging and grading systems available for use in clinical research. These systems can also guide the evaluation of the liver biopsy in daily practice, although there should be clear communication between the pathologist and the clinical staff about which system is being reported.
The first system published was that of Brunt et al., who proposed a system for grading and staging NASH (Table 12.1) [67]. A prerequisite for applying this system was a diagnosis of steatohepatitis, after which the degree of steatosis, portal and lobular inflammation, and ballooning could be combined to provide a final grade of mild, moderate, or marked activity. Fibrosis in this system was staged from 0 to 4 as shown in Table 12.1. Several years later, when the NASH Clinical Research Network was formed, the pathologists in the network were tasked with creating a scoring system that could be used in the natural history studies and clinical trials of the network [68]. The system was an intellectual successor to the Brunt system but extended it so that it could be applicable to any patient, adult or child, that the network might enroll. Selected parts of the system are shown in Table 12.1. An aggregate score, the NAFLD Activity Score (NAS), was defined as the unweighted sum of the steatosis, lobular inflammation, and ballooning scores based on the fact that those features were most closely associated with the diagnosis of NASH. Fibrosis was not included in this score so that the activity score could be distinct from the stage. The aggregate score has proved useful in clinical trials and other clinical studies to demonstrate histological improvement and to correlate with other findings [24, 63, 69, 70]. Patients in the PIVENS clinical trial who showed improvement in NAS also showed improvement in fibrosis [71].
Table 12.1
Central elements of three grading and staging systems for NAFLD and NASH
Feature | Grade/score | Grading/staging system | ||
|---|---|---|---|---|
Brunta [67] | SAF [72] | |||
Steatosis | 0 | None | <5 % | <5 % |
1 | ≤33 % | 5–33 % | 5–33 % | |
2 | 33–66 % | 33–67 % | 33–67 % | |
3 | >66 % | >67 % | >67 % | |
Lobular inflammation | 0 | No foci | No foci | No foci |
1 | 1–2 foci per ×20 field | <2 foci per ×20 field | <2 foci per ×20 field | |
2 | 2–4 foci per ×20 field | 2–4 foci per ×20 field | >2 foci per ×20 field | |
3 | >4 foci per ×20 field | >4 foci per ×20 field | ||
Ballooning | 0 | None | None | Only normal hepatocytes |
1 | Mild, zone 3 | Few | Few: clusters of hepatocytes with rounded shape and reticulated cytoplasm | |
2 | Prominent, zone 3 | Many | Many: enlarged hepatocytes (≥2× normal) | |
3 | Marked, zone 3 | |||
Portal inflammation | 0 | None | None | |
1 | Mild | Mild | ||
2 | Moderate | More than mild | ||
3 | Severe | |||
Fibrosis (stage) | 0 | None | None | None |
1 | Zone 3 perisinusoidal fibrosis only | Perisinusoidal or periportal fibrosis; 3 substages defined | Perisinusoidal or periportal fibrosis | |
2 | Zone 3 perisinusoidal fibrosis and periportal fibrosis | Perisinusoidal and periportal fibrosis | Perisinusoidal and periportal fibrosis | |
3 | Bridging fibrosis | Bridging fibrosis | Bridging fibrosis | |
4 | Cirrhosis | Cirrhosis | Cirrhosis | |
The Fatty Liver Inhibition of Progression (FLIP) consortium has adopted a new system for use in its studies [59]. This system, first published by Bedossa et al., also assesses steatosis, lobular inflammation, ballooning, and fibrosis (Table 12.1) [72]. The scores can be abbreviated for reporting purposes in “SAF” form, where each letter is followed by a number indicating steatosis (S) grade, activity (A), and fibrosis (F) stage. Activity is defined as the sum of the lobular inflammation and ballooning scores. An algorithm, shown in Table 12.2, relates histological findings and scores to diagnosis. Because the SAF system simplifies the relationship between scores and diagnosis, the reproducibility of diagnostic categorization is enhanced [72]. Although other systems have been published [73, 74], they have not garnered the attention of the ones noted above. Because the existing systems (even those not described in detail here) are similar, the selection of one system or another should depend mainly on the familiarity of the pathologist with the system and on the purpose for which it is to be used. All of these systems are semiquantitative in nature and could be supplemented in clinical research with quantitative image analysis, particularly for quantification of steatosis and fibrosis.
Steatosis | Ballooning | Lobular inflammation | Diagnosis |
|---|---|---|---|
0 | 0, 1 ,or 2 | 0, 1, or 2 | Not NAFLD |
1, 2, or 3 | 0 | 0 | NAFL |
1 | NAFL | ||
2 | NAFL | ||
1 | 0 | NAFL | |
1 | NASH | ||
2 | NASH | ||
2 | 0 | NAFL | |
1 | NASH | ||
2 | NASH |
Alcoholic Liver Disease
Introduction
Alcoholic liver disease (ALD) is the third most common risk factor for disease and disability worldwide (World Health Organization, Global Health Observatory. Prevalence of alcohol use disorders. United States: World Health Organization, 2004). Almost 4 % of all deaths in the world result from ALD [75]. Within the spectrum of chronic liver diseases, ALD constitutes the leading cause of liver cirrhosis, the most common cause of hepatocellular carcinoma (HCC) in the Western countries [76] and the second most common indication for liver transplantation [77].
The histological spectrum of ALD is multifaceted but can be summarized in three main schematic patterns: steatosis, alcoholic hepatitis, and fibrosis/cirrhosis. These patterns are not distinct entities but rather a spectrum of overlapping injuries that can be simultaneously present in different combinations. Most of the lesions are shared with NAFLD but may vary with respect to background physiopathology, severity, and prognosis. Indeed, it is accepted that the overall histopathological appearance is usually milder in NASH than in ASH. However, since the histological changes are similar in both diseases, clinical correlation is of utmost importance in helping to define the exact etiology. Finally, there are some additional features that have been described in ALD but not in NAFLD so far and which will be also emphasized in this chapter. Table 12.3 underscores the main pathologic features that may help to orient the diagnosis.
Table 12.3
Similarities and differences in histological features of NAFLD and ALD
Present both in ALD and NAFLD | Evocative of ALD | Evocative of NAFLD |
|---|---|---|
Zone 3 predominance | Sclerosing hyaline necrosis | Glycogenated nuclei |
Lobular inflammation | Extensive neutrophilic infiltration | Predominant lobular mononuclear cell infiltrate |
Macrovesicular steatosis (may be absent in advanced stages) | Alcoholic foamy degeneration | Ductular proliferation |
Portal inflammation (optional) | Venous phlebitis (portal or central vein) | Predominance of periportal lesions in a subset of patients |
Hepatocellular ballooning | Phlebosclerosis of central vein | |
Mallory–Denk bodies | Cholestasis (canalicular or ductular) | |
Apoptotic bodies | Pericholangitis | |
Perisinusoidal fibrosis | Severe steatohepatitis with abundant Mallory–Denk bodies |
The diagnosis of ALD relies on evidence of liver disease in combination with significant alcohol intake and in absence of other comorbidities. Although liver biopsy remains the standard for assessing the type and extent of liver damage, there is a lack of consensus about performing liver biopsy in patients suspected of ALD, given concerns regarding the risk of sampling error and the related safety of liver biopsy [78]. The recent EASL guidelines state that liver biopsy should not be performed in all patients with ALD but is indicated to confirm the diagnosis and to assess the severity of the disease in cases of aggressive forms of ALD requiring intervention and in situations where cofactors may be contributing to the onset of liver disease [79]. Moreover, liver biopsy is useful in determining the outcome of patients affected by ALD, given that a histological diagnosis of steatohepatitis or cirrhosis is associated with an increase in mortality of at least 50 %, in comparison with simple alcoholic steatosis [80].
Steatosis
Steatosis is the earliest and most common manifestation of alcoholic liver disease and is seen in up to 90 % of ALD patients [81]. The accumulation of lipid droplets containing primarily triglycerides, the natural end product of fatty acid metabolism, but also free fatty acids and other components within the hepatocellular cytoplasm is generally asymptomatic or may be associated by mild disturbance of liver function tests. This lesion is considered quickly reversible with alcohol abstinence. Although a minimum of 5 % hepatocytes containing lipid vacuoles is required for the diagnosis of steatosis in NAFL, this is not necessary in the context of ALD. Steatosis occurs predominantly in hepatocytes adjacent to the terminal hepatic venule (acinar zone 3). As the liver injury progresses, steatosis can be seen diffusely throughout the lobule. When steatosis is massive, the liver is often enlarged and smooth in addition to having a characteristic yellow appearance on gross examination.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






