Chapter 3 Pancreatic physiology and functional assessment
The Pancreas
The pancreas is a complex organ. In addition to nerves, blood vessels, and connective tissue, it is composed of endocrine and exocrine elements. The endocrine pancreas consists of 105 to 106 islets of Langerhans, each of which contains approximately a thousand endocrine cells. Together the islets constitute approximately 2% of the normal human pancreas. The exocrine pancreas includes the acinar cells, which account for 75% to 90% of the pancreatic mass, and the duct cells, which account for approximately 5%. Most of the currently available evidence indicates that all endocrine and exocrine pancreatic cells are derived from a common stem or progenitor cell (Fishman & Melton, 2002; Zaret & Grompe, 2008). Commitment to become either a duct cell or an acinar cell is believed to occur relatively early during embryogenesis. Subsequently, the endocrine cells undergo terminal differentiation into islet and acinar cells, but some also still may be capable of differentiating into duct cells (Gu et al, 2003). Conversely, some duct cells may be capable of differentiating into endocrine cells.
Endocrine Pancreas
Structure and Innervation
The islets of Langerhans are composed of four different cell types evenly distributed throughout the pancreas. Approximately 15% of islet cells are α cells, which secrete glucagon; 70% to 80% are β cells, which secrete insulin; 5% to 10% are δ cells, which secrete somatostatin; and 15% to 25% are pancreatic polypeptide (PP) cells, which secrete pancreatic polypeptide (Kulkarni, 2004). The PP cell type is only sparsely present in the dorsal pancreas, neck, body, and tail; most PP cells are found in the portion of the pancreas derived from the ventral pancreatic bud (the head and uncinate process). Rodent islets consist of a core composed of β cells surrounded by the other cell types, but in humans, the various cell types are intermixed within each islet. These islets contain a rich capillary network, and they receive substantial blood flow.
The islets of Langerhans are innervated by parasympathetic and sympathetic nerves. Vagal stimulation increases insulin release, and vagal stimulation may play a key role in mediating the cephalic phase of insulin secretion; that is, insulin release in response to the sweet taste of glucose. Although a cephalic phase of insulin secretion has been shown to occur in rats and dogs, it is not certain that a cephalic phase occurs in humans. Stimulation and inhibition of insulin release have been associated with sympathetic nervous stimulation in experimental animals, but the role of sympathetic nerves in regulating insulin release in humans has not been established (Nauck, 1998).
Synthesis and Storage of Insulin
Insulin is assembled within the endoplasmic reticulum as preproinsulin (Madsbad et al, 1992; Malaisse, 1992; Steiner & James, 1992). With cleavage of a signal sequence, the intermediate product proinsulin is formed, packaged within Golgi-derived vesicles that evolve into immature storage granules and eventually become mature secretory granules. During this process of granule maturation, proteolytic processing of insulin continues, liberating mature insulin from its C-peptide. Mature insulin, its C-peptide fragment, and other processing intermediates are stored together within the secretory granules, until they are all discharged at the cell surface. Normally, that secretion occurs primarily in response to external stimuli (regulated secretion). In insulinomas, insulin and its processing intermediates often are released continuously, however, without the need for external stimuli (constitutive secretion; see Chapter 61).
Stimulus–Secretion Coupling for Insulin Secretion
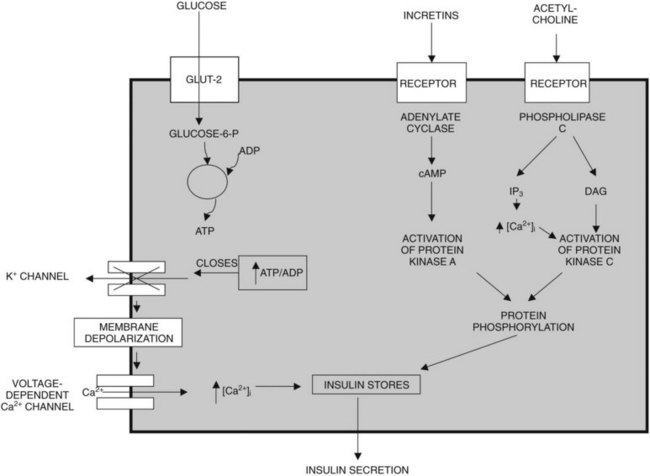
Glucose, certain amino acids (e.g., arginine), acetylcholine, and incretins such as gastric inhibitory peptide (GIP) and glucagon-like peptide (GLP-1) are the most important regulators of insulin release by β cells (Nauck, 1998). To varying degrees, the intracellular mechanisms responsible for insulin release in response to each of these types of regulators are different (Fig. 3.1). The “first phase” of insulin release seems to involve mechanisms that differ from the mechanisms in the later phase of sustained insulin secretion. This review focuses on events believed to underlie that first phase of insulin secretion (Rutter, 2001).
Glucose, the most important regulator of insulin release, enters β cells via a highly efficient glucose transporter, GLUT-2, which for the most part equalizes intracellular and extracellular glucose concentrations (Unger, 1991). Subsequent to its uptake by the cell, glucose is phosphorylated by glucokinase (Meglasson & Matschinsky, 1986), which, because of its high michaelis-menten constant (Km), acts as a glucose sensor. Glucokinase catalyzes the formation of glucose-6-phosphate, and as a result, glucose-6-phosphate accumulates within the cell at concentrations closely related to the intracellular concentration of glucose. Glucose-6-phosphate is metabolized, leading to formation of adenosine triphosphate (ATP); this results in an increased ATP–ADP (adenosine diphosphate) ratio, which causes closure of an ATP-dependent potassium channel in the plasma membrane of β cells (Henquin, 1988). Closure of this channel results in membrane depolarization, which leads to activation of voltage-dependent L-type calcium channels and calcium influx into the cell (Misler et al, 1992). The resulting increase in cytoplasmic calcium levels triggers exocytosis of the secretory granules that contain insulin (Eliasson et al, 2008). In contrast to the initial phase of insulin secretion, it is believed that the later phase of sustained secretion does not involve closure of ATP-dependent potassium channels (Rutter, 2001).
Amino acids such as arginine also upregulate insulin secretion by being metabolized and causing ATP levels to increase, increasing the ATP–ADP ratio and triggering calcium influx. The incretins, including GIP and GLP-1, are believed to upregulate insulin secretion by activating adenylate cyclase, increasing cyclic adenosine monophosphate (cAMP) levels, activating protein kinase A, and causing phosphorylation and activation of exocytosis-related proteins; cAMP also may promote insulin secretion by mechanisms that involve activation of L-type calcium channels and accelerated calcium influx. Insulin secretion also can be stimulated by acetylcholine released from cholinergic nerves. Acetylcholine binds to receptors on the cell surface, causing activation of phospholipase C and generation of the secondary messengers, inositol triphosphate (IP3), and diacylglycerol (DAG). IP3 triggers release of calcium (Ca2+) from intracellular storage pools, which causes release of insulin. DAG binds to and activates protein kinase C, which, like protein kinase A, promotes insulin secretion by phosphorylating and activating exocytosis-related proteins (Persaud et al, 1989; Prentki & Matschinsky, 1987).
Incretins and the Regulation of Insulin Secretion
Insulin secretion in response to orally administered glucose and other nutrients is 25% to 50% greater than that after intravenous administration (Creutzfeldt, 1979; Tillil et al, 1988). This so-called incretin effect is mediated by hormones released from the gut, primarily GIP and GLP-1 (Fiesler et al, 1995; Nauck et al, 1993). GIP is produced by K cells in the duodenum and upper jejunum, and it is released into the circulation after ingestion of nutrients. GLP-1 is synthesized in enteroglucagon or L cells, which also contain peptide YY (PYY) and enteroglucagon. These L cells are primarily located in the distal small bowel, colon, and rectum. Of the two hormones GIP and GLP-1, GIP is probably the major physiologic incretin. Glucose and amino acids are considered to be “primary” or “direct” stimulants of insulin secretion, but insulin secretion is also modulated by the incretins. These incretins cause little or no insulin release in the absence of glucose or amino acids, but they can alter insulin release dramatically in response to either glucose or amino acids.
Glucagon and Other Islet Hormones
In addition to insulin, the islets of Langerhans secrete somatostatin, pancreatic polypeptide, glucagon, and possibly ghrelin. Clinically, pancreatic diseases generally are not associated with disorders of somatostatin, because somatostatin is synthesized and released by many other organs besides the pancreas. Pancreatic polypeptide, released from the ventral region of the pancreas in response to food, does not seem to have any specific physiologic function, and as a result, abnormal pancreatic polypeptide secretion is not associated with clinically recognizable abnormalities. Glucagon, released from islet α cells, is a functionally important hormone, and disorders of glucagon secretion can result in clinically recognizable functional changes (Lefebvre, 1992).
Glucagon is a 29–amino acid hormone that functions to regulate hepatic glucose output. Acting via adenylate cyclase and cAMP, glucagon stimulation of hepatocytes accelerates glycogenolysis and gluconeogenesis, causing blood glucose levels to increase. Hypoglycemia promotes glucagon release, and hyperglycemia inhibits glucogon release (Lefebvre, 1992; Unger, 1985). In some species, glucagon has other effects, including accelerating lipolysis, promoting gastric emptying, and stimulating insulin secretion. In type 1 diabetes, there is a low insulin–glucagon ratio, and this worsens hyperglycemia by promoting hepatic glucose output. In type 2 diabetes, glucagon secretion seems to be increased, and it is not suppressed by hyperglycemia. As a result, plasma glucagon levels are normal or elevated. With total pancreatectomy, patients become profoundly glucagon deficient and consequently prone to hypoglycemia. This leads to so-called brittle diabetes, which is characterized by swings in blood glucose levels, from marked hyperglycemia to marked hypoglycemia.
Endocrine Pancreas in Diabetes
Type 1 diabetes is a chronic autoimmune disease that leads to the destruction of β cells (Powers & Eisenbarth, 1995). Consequently, disordered insulin secretion in type 1 diabetes is primarily related to progressive loss of β cells, and clinical diabetes occurs when 80% to 90% of β cells are destroyed. In type 1 diabetes, serum insulin and C-peptide levels are usually extremely low (Polonsky et al, 1986). In contrast, the islets of Langerhans usually appear histologically normal in patients with type 2 diabetes, or the cells may be normal, but the islet may contain deposits of islet amyloid polypeptide. Despite this appearance, however, β-cell mass may be reduced by 50% in patients with type 2 diabetes, especially in patients who require insulin therapy. For the most part, patients with type 2 diabetes secrete increased amounts of insulin, proinsulin, and insulin conversion intermediates such as C-peptide, and elevated circulating levels of these factors can be detected (Porte, 1991).
Endocrine Pancreas in Chronic Pancreatitis
In chronic pancreatitis, there is progressive scarring of the pancreas with replacement of the gland by fibrous tissue. The islets become embedded in these areas of fibrosis, and eventually islets and islet cell mass are lost. This loss leads eventually to the loss of β cells and α cells (Diem, 2002). Functional testing may reveal a reduced response to oral and intravenous glucose and to glucagon and arginine. Approximately 10% of patients with exocrine pancreatic insufficiency secondary to chronic pancreatitis have clinically significant diabetes, whereas 30% have impaired glucose tolerance (Larsen et al, 1987; Sjoberg & Kidd, 1989).
Exocrine Pancreas
On a tissue-weight basis, the exocrine pancreas synthesizes and secretes more protein per day than any tissue in the body other than the lactating breast, and approximately 90% of that newly synthesized protein consists of digestive enzymes. On a daily basis, the normal human pancreas secretes 6 to 20 g of digestive enzyme protein in 2.5 L of bicarbonate-rich fluid (Case, 1998). Derangements of exocrine pancreatic function commonly are observed in patients with pancreatic disease, and pancreatic functional assessment mostly involves tests of exocrine pancreatic function. The structure of the exocrine pancreas, its neurohormonal control, and the cell physiology of exocrine pancreatic secretion are reviewed in this section, and when possible, this review emphasizes information known to be valid for humans. Most of the literature concerning these issues is based, however, on work performed using various nonprimate species—rats, mice, guinea pigs, cats, rabbits, dogs, and pigeons—or work performed in vitro using isolated cells. To a great extent, this literature has indicated that there may be important differences in pancreatic exocrine function and physiology among the various species as well as important differences between the in vivo and in vitro setting. Although many of the overall concepts covered in this section are no doubt relevant to the human in vivo setting, there may be important exceptions to this generalization (Case, 1998).
Structure
The exocrine pancreas is composed of two functional elements: a ductal tree and a mass of pancreatic acini (Case, 1998; Konturek et al, 2003). Each acinus consists of a grapelike cluster of acinar cells that surround a central or acinar lumen, which is continuous with the ductal space. Acinar cells constitute 75% to 90% of the gland. They are highly polarized cells that possess abundant, rough endoplasmic reticulum, located almost entirely within the basolateral region of the cell. The nucleus also is located in the basolateral region, and a prominent Golgi complex is located near the center of the cell. Secretory vesicles consisting of zymogen granules and their immature condensing vacuole precursors fill the apical portion of acinar cells.
The pancreas is innervated by extrinsic and intrinsic parasympathetic and sympathetic nerves. These autonomic nerves have been shown to use a vast array of neurotransmitters in addition to acetylcholine and catecholamines, including nitric oxide, vasoactive intestinal peptide (VIP), gastrin-releasing peptide, neuropeptide Y, galanin, substance P, calcitonin gene-related peptide, gastrin/cholecystokinin (CCK), and enkephalins (Case, 1998; Case & Argent 1993). From a purely functional standpoint, the most important of the neurotransmitters seems to be acetylcholine, which in addition to functioning as a neurotransmitter also acts as a paracrine hormone released near acinar cells to regulate their function. Evidence indicates that many of the intrapancreatic cholinergic vagal nerves possess CCK receptors, and that at least in humans, the hormonal effects of CCK on pancreatic acinar cells may be mediated by vagal afferents in response to CCK stimulation (Owyang & Logsdon, 2004). Whether or not CCK can directly stimulate acinar cell secretion of enzymes in humans is a controversial issue (Murphy et al, 2008; Owyang & Logsdon, 2004).
Neurohormonal Regulation of Exocrine Pancreatic Function
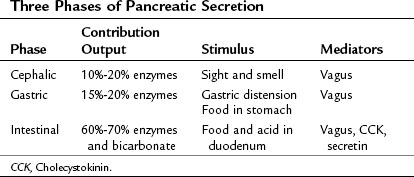
The exocrine pancreatic response to a meal traditionally is considered to involve three phases (Konturek et al, 2003): 1) the cephalic phase, triggered by the sight or smell of food, accounts for 10% to 20% of the pancreatic secretion during a meal; 2) the gastric phase, triggered by food entering the stomach and by gastric distention, accounts for 15% to 20% of meal-stimulated pancreatic secretion; and 3) the intestinal phase, triggered by food and acid entering the duodenum and proximal jejunum, accounts for 60% to 70% of meal-stimulated pancreatic secretion (Table 3.1). In humans, the cephalic and gastric phases are thought to be vagally mediated and to primarily involve stimulation of digestive enzyme secretion rather than bicarbonate secretion (Anagnostides et al, 1984; Case, 1998; Konturek et al, 2003). The intestinal phase is neurally and hormonally regulated, however, and it involves secretion of a pancreatic juice that is rich in bicarbonate and digestive enzymes.
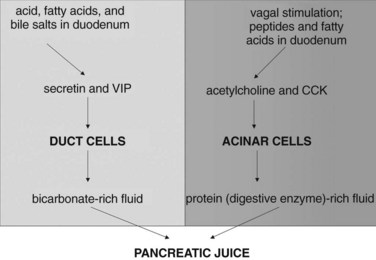
The intestinal phase of meal-stimulated pancreatic secretion has been the most fully characterized phase (Fig. 3.2). The entry of gastric chyme into the duodenum and proximal jejunum triggers vagovagal (centrally acting) reflexes and enteropancreatic neural reflexes that together primarily stimulate digestive enzyme secretion from pancreatic acinar cells. Acidification of the duodenum (pH <4.5) causes specialized cells within the mucosa to release secretin into the circulation, and secretin potently stimulates duct cell secretion of fluid and bicarbonate (Case & Argent, 1993). Release of secretin and stimulation of fluid/bicarbonate secretion also are elicited by various products of digestion, especially fatty acids and bile salts (Rhodes et al, 1988; Riepl et al, 1990). Fatty acids and polypeptides released by the partial digestion of food also stimulate release of CCK from other specialized cells within the duodenal and jejunal mucosa, and elevated CCK levels cause acinar cells to secrete digestive enzymes and other proteins into the pancreatic juice (Dale et al, 1989).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree