Chapter 50 Pancreatic Duct Leaks and Pseudocysts
Introduction
The initial manifestations of acute pancreatitis are caused for the most part by local enzyme activation and acute cytokine release. This combination leads to local pain, ileus, peripancreatic burn, systemic inflammatory response syndrome, and early organ failure including acute respiratory distress syndrome. Perpetuation of the disease process may be a consequence of infection of necrotic tissue or ongoing ductal leak.1–4 Chronic pancreatitis may also result in a pancreatic duct leak or fistula, as may trauma, surgical or otherwise.1,5 In chronic pancreatitis, the consequence of the leak depends on the etiology, the size of the ductal disruption, the location of the leak relative to anatomic tissue planes, and the body’s success in walling off and containing the disruption. In traumatic pancreatitis, there is usually a smoldering acute inflammatory response and an acute leak. This combination can result in a seriously ill patient after penetrating trauma or a patient who remains clinically well after surgical drain placement at the time of splenectomy and inadvertent damage to the pancreatic tail.6
Pancreatic duct leaks or fistulas have traditionally been defined as internal or external.3,7 External leaks (pancreaticocutaneous fistulas) almost always follow percutaneous drainage of internal pancreatic fluid collections or pancreatic surgery. Less commonly, they are the consequence of penetrating abdominal trauma. Internal pancreatic fistulas include pancreaticoenteric fistulas, pseudocysts, pancreatic ascites, and pancreatic pleural effusions.4,8 Pancreatic necrosis, which is clearly associated with ductal disruption in three-fourths of patients, has not traditionally been defined as the cause or consequence of a pancreatic fistula.9–11 Box 50.1 summarizes the current classification of pancreatic fistulas.
Epidemiology
The incidence of pancreatic duct leaks is uncertain and seems to be independent of the cause of the underlying pancreatitis. Whether caused by alcohol, biliary tract disease, metabolic disorders, or medications, an acute leak seems to be related more to disease severity. Multiple reports suggest that 30% to 75% of pancreatic necrosis is associated with ductal disruption, although there is considerable debate whether this disruption is a primary or secondary phenomenon.7,9,10,12 Also, 40% of patients with acute pancreatitis may develop some peripancreatic fluid collection, although less than 5% of these patients develop a true pseudocyst, and a much smaller percentage have decompression of these fluid collections by formation of a pancreaticoenteric fistula.13 Chronic pancreatitis predisposes not only to pseudocyst formation but also to pancreatic ascites and high-amylase pleural effusions. High-amylase pleural effusions are chronic and have a distinctly different chemical composition and pathophysiology than the more common acute pleural effusions noted in the setting of severe acute pancreatitis.3,7
Pathogenesis
Pancreatic duct leaks are the consequence of enzyme activation with subsequent necrosis of ductal epithelium, the result of increased intraductal pressure often behind a stricture or stone or both.3,7 Alternatively, leaks may be caused or perpetuated by percutaneous drainage of peripancreatic fluid collections; surgical resection or bypass; tumor disruption of ductal epithelia; or pancreatic trauma, particularly penetrating trauma.14–25 Box 50.2 lists some etiologies of pancreatic duct leaks.
Clinical Features
The clinical features of pancreatic duct leaks depend on the cause of the disruption and its size and site. Pancreatic juice follows tissue planes, and the body is variably successful in containing this leak contingent on such factors as rate of leak and presence or absence of superinfection. Superinfection, early cytokine release, bacterial translocation from the gut, endotoxin release, and extraluminal enzyme activation also determine many of the clinical features associated with acute pancreatitis, such as pain, ileus, nausea and vomiting, tachycardia, oliguria, and hypotension.26,27 From an anatomic standpoint, a leak may be low grade and stay within the confines of the parenchyma leading to smoldering pancreatitis or variable degrees of necrosis, the latter often associated with multisystem organ failure and local and systemic infections.7,28–32 Necrosis may also lead to internal fistulization into contiguous organs including the C-loop most commonly but also the bile duct, stomach, transverse colon, or jejunum.3,33–36
Depending on the degree of leak and its perpetuation by necrosis or downstream ductal obstruction and ongoing oral feeding and pancreatic stimulation, head leaks often are associated with right pararenal fluid collections and can track along the psoas musculature to cause pelvic fluid collections that can track into the scrotum or buttocks.36 If volumes of juice are sufficient, with resultant pancreatic ascites, I have even seen prolapsed and ulcerated vaginal vaults and uteri because of increased intraabdominal pressure. Pancreatic head leak that is successfully walled off by the body may cause a pseudocyst localized to the right upper quadrant. Although the latter may be asymptomatic, if small, common presentations of larger pseudocysts in this location include postprandial or chronic pain, early satiety or postprandial nausea and vomiting from variable degrees of gastric outlet obstruction, or biliary obstruction. Biliary obstruction may cause jaundice or occasional cholangitis but is more often associated with liver function abnormalities including variable elevations of transaminases and alkaline phosphatase.
Leaks of the pancreatic duct tail have been associated with left upper quadrant or perisplenic pseudocysts,3,37 if contained and walled off. Alternatively, they may track into the retroperitoneum and cause high-amylase pleural effusions22,38–40 or acute pararenal or pelvic fluid collections. Fistulization into the ligament of Treitz or the transverse colon or splenic flexure also is occasionally seen but almost exclusively in the setting of active necrosis.15,34,41 Depending on the rapidity of the leak and the presence or absence of concomitant necrosis, clinical signs and symptoms of a tail leak may include shortness of breath, nausea and postprandial pain, or clinical signs of sepsis because of a pancreaticocolonic fistula.
Leaks that occur from the genu to the distal body or proximal tail area of the pancreas occur most commonly in the setting of necrosis and result in lesser sac fluid collections.3,14,42–46 Traditionally defined as pseudocysts, these fluid collections are usually more complex, containing considerable saponified fat and tissue debris. The consistency and viscosity of lesser sac fluid collections are routinely misinterpreted by abdominal computed tomography (CT), often leading to therapeutic misadventures with attempts to drain these collections radiographically, endoscopically, or surgically.4,11,19,47–50 To distinguish these collections from more traditional pseudocysts that can have a similar imaging appearance, Baron and colleagues50 termed these latter collections evolving pancreatic necrosis, a variant of walled-off pancreatic necrosis, and suggested that the patient’s clinical course may be more important than traditional abdominal imaging.
The lesser sac is also often a decompressive site for patients with chronic pancreatitis with downstream duct obstruction from a pancreatic stone or stricture. This condition can result in a variably sized pseudocyst or pancreatic pericardial effusion if there is mediastinal involvement. Additional chest manifestations include pancreatic pleural effusion, as noted previously, and pericardial tamponade or pancreaticobronchial fistulas.3,31 Central pancreatic leaks are usually the cause of pancreatic ascites also.7,22,39,51 Associated with a concomitant and leaking pseudocyst in 50% of patients, clinical presentation may include increased pain plus abdominal girth, shortness of breath from diaphragmatic compression or concomitant pleural effusions, and occasional spontaneous bacterial peritonitis from bacterial translocation from the gut.
Pathology
Because of the variability of etiology of ductal disruptions, there is no one all-encompassing pathology. Instead, chronic pancreatitis is usually associated with the formation of a leak and its myriad manifestations (pseudocyst, ascites, and pancreatic pleural effusions) by virtue of ductal obstruction by an inflammatory stricture or intraductal calcification.3,8 In such settings, acute parenchymal inflammation may be negligible. In contrast, the acute inflammatory response seen in acute pancreatitis, particularly pancreatic necrosis, has been claimed by some authors to be the primary event with subsequent lysis of ductal epithelial cells resulting in a leak.52 There is likely a mixture of scenarios in either setting with the resultant pathology depending on the site and size of the disruption; the presence or absence of activated enzymes; and the body’s success at walling off the leak, initially with inflammatory cells but later with formation and organization of collagen. The last-mentioned is perhaps best represented by a pseudocyst that can be broken down further into acute pseudocyst (collection of pancreatic juice enclosed by a wall of nonepithelialized granulation tissue that arises as a consequence of acute pancreatitis, requires at least 4 weeks to form, and is devoid of significant solid debris) and chronic pseudocyst (a collection of pancreatic juice enclosed by a wall of fibrous or granulation tissue that arises as a consequence of chronic pancreatitis).23,50
Differential Diagnosis
The etiology and the benign nature of most pancreatic duct leaks are easy to confirm if one considers the diagnosis in the first place because of access to excellent abdominal imaging through ultrasound, CT scanning, magnetic resonance (MR) imaging including secretin-magnetic resonance cholangiopancreatography (S-MRCP), and endoscopic modalities such as endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP).3,13,23,53–57 Routine aspiration of ascites or pleural effusions for amylase and lipase usually confirms a pancreatic etiology, and a fluid collection in the left upper quadrant after a splenectomy, left nephrectomy, or complicated antireflux procedure can be confirmed as pancreatic in origin if one thinks to check an amylase level at the time of diagnostic percutaneous aspiration or therapeutic drain placement. In these instances, inadvertent damage to the tail of the pancreas is substantially more common than local perforation of the stomach or splenic flexure of the colon at the time of surgery.
In addition, the diagnosis of a pancreatic duct leak should not be difficult in patients with a persistent fluid output after a pancreatic resection or percutaneous drainage of an acute, amylase-rich fluid collection in the setting of acute or chronic pancreatitis. The major differential diagnostic dilemma occurs in patients without a known history of pancreatitis who present with what appears to be a pseudocyst. There have been multiple approaches to distinguish pseudocysts from cystic neoplasms and benign from potentially malignant cystic tumors. Ultrasound and CT characteristics favoring pseudocyst include parenchymal or ductal calcifications, a uniform appearance to the cyst, and lack of calcifications in the lesion itself. Cysts that show an irregular wall thickness with mass effect, septations, or punctate wall calcification are more likely to be neoplastic. Cyst aspiration for amylase, mucus, carcinoembryonic antigen level, and cytology can be done under CT, ultrasound, or EUS guidance and has been used to distinguish benign and malignant neoplastic cysts from pseudocysts.58 Pseudocysts are discussed in detail in other chapters.
As noted previously, S-MRCP has occasionally been used to document a pancreatic duct leak, particularly in the setting of pancreatic necrosis.53 ERCP has been used more commonly not only to diagnose but also to treat pancreatic duct leaks. Leaks may be demonstrable by abnormal flow of contrast material into a pseudocyst, into the peritoneal or thoracic cavity, or into the bile duct or a contiguous loop of bowel in the setting of internal fistulas.1,3,7 Alternatively, contrast material can often be seen flowing into a surgically or radiologically placed Jackson-Pratt drain in external fistulas.5,22,36,59 In the setting of central pancreatic necrosis or severe chronic pancreatitis, ERCP may simply document a complete obstruction of the main pancreatic duct. In this setting, the leak occurs upstream from the obstruction or from a disconnected portion of the gland—the disconnected duct syndrome. Box 50.3 summarizes some diagnostic tests available for pancreatic duct leaks.
Treatment
Therapy for pancreatic duct leaks does not occur in an endoscopic vacuum. Strategies include preventing a leak in the first place, using good surgical technique, and possibly using intraoperative fibrin glue or stent placement or postoperative octreotide after partial pancreatectomy or decompressive pancreatic surgery.5,60–64 The ability to place a transpapillary stent does not mean that this modality is suitable for all patients. Individuals with leaks are best approached by a team consisting of an interventional radiologist, a pancreaticobiliary surgeon, and an endoscopist capable of performing both diagnosis and therapy (Box 50.4).1,3,65
Pseudocysts
Pseudocysts were historically treated surgically, usually by cyst-enteric or cyst-gastric anastomoses, although pancreatic resection has occasionally been used for pseudocysts in the pancreatic tail.25,66–69 Likewise, complex cysts with significant internal septations or debris have been treated with external drainage. Morbidity and 30-day mortality rates for open surgery have approximated 25% to 30% and 2% to 5% with recurrence rates of 10% to 20%.25,57,69,70 These statistics have led some centers to approach surgical decompression laparoscopically and to insist on preoperative MRCP or ERCP to delineate better the ductal anatomy as a guide to type of surgery (decompression vs. resection).25,56,71,72 No randomized trials have compared surgical with nonsurgical management of pseudocysts; instead, retrospective reviews are available in which 30 patients with pseudocysts were treated with surgery (49%), endoscopy (39%), or percutaneously (11%). There were no differences in pseudocyst resolution or complications in patients treated surgically or endoscopically.73
A series by Melman and colleagues66 defined higher initial success rates for laparoscopic versus open pseudocyst drainage, although comparable success rates were noted with endoscopic, radiologic, or salvage surgical drainage. In many centers, percutaneous drainage of pseudocysts with long-term catheter placement has become the standard of care by which other treatment modalities have been judged. Individual series and meta-analyses of the literature suggest 85% successful resolution rates, although catheter occlusions with subsequent bacterial seeding and iatrogenic infection with need for urgent catheter exchange remain problematic particularly if the cyst is filled with debris.7,36,74 In addition, in individuals who develop a disconnected gland syndrome from trauma or necrosis, placement of a percutaneous drain may result in a chronic pancreatic external fistula that may necessitate Jackson-Pratt drainage for months or years. Alternatively, percutaneous injection of glue or fibrin has been used in an attempt to close the fistulous tract, and surgery may be required to resect the distal (tail), disconnected portion of the gland.75
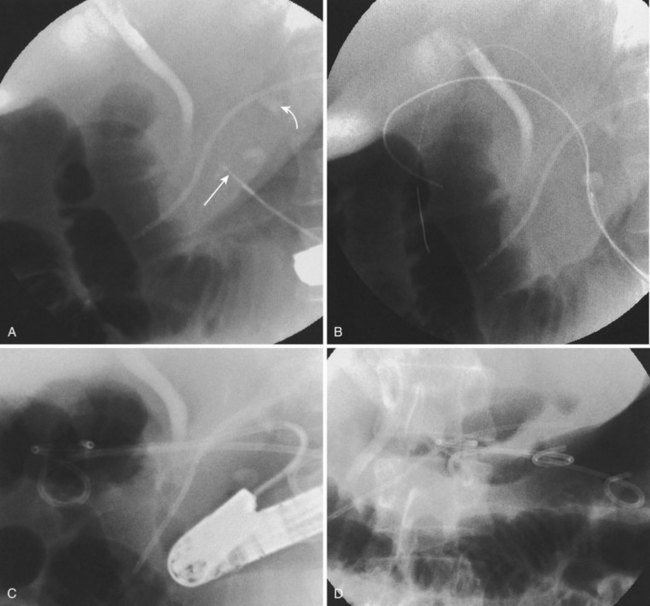
Endoscopic pseudocyst drainage was first described by Rogers and coworkers76 in 1978 using a needle placement through the gut wall to drain a pseudocyst that rapidly recurred. The first successful electrocautery fistulization into a pseudocyst was done more than 2 decades ago and resulted in permanent cure in three of the first four patients in whom it was undertaken.77 Although the procedure has been refined to take advantage of abdominal CT, EUS, and MR imaging and MRCP, large pseudocysts still require some form of access, either by needle-knife sphincterotome or transgastric or transenteric injection with a Seldinger needle followed by placement of one or more guidewires into the cavity proper (Fig. 50.1).78–97 Historically, the incisions were enlarged using some form of electrocautery (conventional or needle-knife sphincterotomy or an overtube that conducted cautery), but 6- to 10-mm hydrostatic balloons are used in most cases at the present time to enlarge the communication to the stomach or duodenum. A variation of this procedure is use of a transluminal balloon accessotome.98 Although various stents have been used to maintain the fistulous communication between the gut and pseudocyst, most endoscopists currently use 7-Fr to 10-Fr double-pigtail stents to minimize migration, leaving them in place for 6 to 8 weeks or until abdominal imaging has confirmed pseudocyst resolution.
Although the need for preprocedure antibiotics has not changed, other things have. With the advent of therapeutic EUS scopes, it is no longer necessary to see a “bulge” on the stomach or duodenal wall to ensure that one is entering the fluid collection.83,89–91,94,95,99–102 Therapeutic duodenoscopes are not required; concomitant ERCP is usually employed to define ductal anatomy including the presence of an ongoing leak or disconnected duct and gland syndrome.1,7 A leak can be treated with transpapillary stents (Fig. 50.2) allowing resolution of small pseudocysts without need for concomitant drainage102
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree