Chapter 63B Palliation of pancreatic and periampullary tumors
Overview
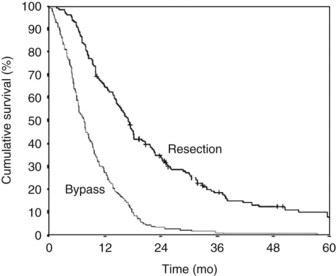
Pancreatic and periampullary tumors are the fifth most common cause of cancer-related death in the Western world (DiMagno et al, 1999; Greenlee et al, 2000). The incidence in the United States is approximately 10 per 100,000 persons per year. Most of these tumors are pancreatic adenocarcinoma, and the survival is poor (DiMagno et al, 1999; Greenlee et al, 2000; Gudjonsson, 1995, 2009; Kuhlmann et al, 2004). Despite surgical treatment with or without radiotherapy and chemotherapy, the overall 5-year survival is approximately 4% and has barely improved over the last few decades (Fig. 63B.1; Gudjonsson, 2009; Kuhlmann et al, 2004). Most patients are seen initially with “incurable” disease, owing to extensive local disease or metastases at the time of diagnosis (Sener et al, 1999). Confusion surrounds the terminology, however, with the words incurable, inoperable, and unresectable having a variety of interpretations. Use of the term unresectable partly depends on the local surgical philosophy (e.g., accepting a resection of the mesenteric artery portal vein and macroscopically nonradical R2 resections). This surgical philosophy is not only a country-related or regional pattern, it also may be influenced by the experience at the center and the local tradition of the surgeons. The strong relationship between outcome and hospital mortality may play a role in the indication for resection and acceptance of palliative resections (Alexakis et al, 2004; Birkmeyer et al, 2003; Gouma et al, 2000).
It has been questioned whether cure is possible at all in patients with pancreatic cancer (Gudjonsson, 1995, 2009). Gudjonsson summarized the literature, and after adjusting for calculations and duplications, suggested that the total number of 5-year survivors is hardly more than 700 to 800.
There is consensus, however, that patients who undergo resection have the best chance for long-term survival (Alexakis et al, 2004). A recent study showed up to 20 years survival after resection (Adham, 2008). Survival data for patients with distal bile duct carcinoma do not differ from data for patients with pancreatic cancer. Patients with ampullary tumors have a 5-year survival of 20% to 45%, and only a few go straight to palliative treatment (de Castro et al, 2004).
Overall, most patients have palliative treatment, and palliation of symptoms is still the major focus. According to the World Health Organization (WHO, 2002), palliative care is aimed at the improvement of the quality of life (QOL) of patients who face a life-threatening illness and at the prevention or relief of pain and symptoms. The three most important symptoms that should be treated in advanced pancreatic and periampullary cancer are 1) obstructive jaundice, 2) duodenal obstruction, and 3) pain.
The decision to aim for palliative treatment can be made at two different points during the disease (Nakakura & Warren, 2007). The first decision generally is made after staging procedures, and a selection is made for potential curative surgery, palliative surgery, or nonsurgical (endoscopic) palliation. Recently, another option, preoperative radiochemotherapy and subsequent exploration, has been introduced for patients with borderline resectable lesions in an attempt to reduce local tumor ingrowths (Abrams et al, 2009; Bjerregaard et al, 2009).
Accurate initial staging is the crucial step for the selection of surgical and nonsurgical (palliative) treatment. Radiologic imaging techniques have evolved rapidly in the last decade. Various methods, such as contrast-enhanced spiral computed tomography (CT) and endoscopic ultrasonography (EUS), have enhanced the accuracy of radiologic staging, and noninvasive staging procedures are currently the first choice (Alexakis et al, 2004; McMahon et al, 2001; Phoa et al, 2000). Recent evaluation has identified the “new group” of patients, the borderline resectable patients, for pretreatment assessment, and criteria have been described (Callery et al, 2009). Endoscopic retrograde cholangiopancreatography (ERCP) is no longer used as a diagnostic procedure.
The role of diagnostic laparoscopy in combination with laparoscopic sonography has been studied extensively in the past (Bemelman et al, 1995; Friess et al, 1998; Nieveen van Dijkum et al, 2003; Pisters et al, 2001; Tilleman et al, 2004), but its role has diminished. A review showed that no data justify the routine use of staging laparoscopy (Pisters et al, 2001). In a recent series, however, metastasis or local ingrowth was found in 27%. Some authors therefore suggest that it should be used selectively for patients with larger tumors and tumors located in the body and tail (Chang, 2009).
Depending on local philosophy, patients who are found to have a resectable tumor at preoperative noninvasive diagnostic workup should undergo an exploratory laparotomy immediately without preoperative biliary drainage (van der Gaag et al, 2010). Patients with unresectable or incurable disease found during exploration (11% to 50%) are generally considered to be best treated with surgical palliation (Nieveen van Dijkum et al, 2003; Pisters et al, 2001; Sohn et al, 1999). This chapter evaluates the current knowledge of different aspects of surgical and nonsurgical palliative treatment for the symptoms mentioned above: obstructive jaundice, duodenal obstruction, and pain.
Obstructive Jaundice
At the time of diagnosis, 90% of patients with pancreatic and periampullary tumors present with obstructive jaundice. Obstructive jaundice is associated clinically with jaundiced skin and sclerae, nausea, pruritus, dark-colored urine, and decoloring of the stool. More severe consequences are liver dysfunction and eventually hepatic failure secondary to bile stasis and cholangitis, which is found more frequently in patients with ampullary lesions compared with patients with pancreatic cancer. Relief of obstructive jaundice causes a dramatic increase in the QOL of patients and should always be achieved (Abraham et al, 2002; Crippa et al, 2008).
Biliary drainage can be achieved nonsurgically by placement of a biliary stent (endoscopic or percutaneous; see Chapter 18) or surgically by performing a biliary bypass (see Chapter 29). The success rate for short-term relief of biliary obstruction is comparable for surgical and nonsurgical biliary drainage procedures and ranges from 80% to 100%.
In the past, endoscopic biliary drainage was widely performed with plastic stents (polytef [Teflon] and polyethylene). Plastic stents can cause complications such as migration and occlusion, reported in 40%. A new stent type for endoscopic treatment is the self-expandable, covered metallic stent. Compared with plastic stents, expandable stents have a longer patency, but they generally cannot be removed after placement (Davids et al, 1992; Kaassis et al, 2003; Weber et al, 2009).
A surgical bypass can be performed by a hepaticojejunostomy alone or by a double bypass including a gastroenterostomy. In earlier studies, surgical bypass was associated with high rates of morbidity and mortality (Watanapa & Williamson, 1992).
Surgical Palliation of Obstructive Jaundice
Different surgical approaches can be used to obtain adequate biliary drainage. External biliary drainage by a T-tube has been used in the past but results in loss of appetite, and electrolyte and fluid imbalances frequently accompany external drainage. Internal biliary drainage generally is preferred and is performed by a cholecystojejunostomy, choledocho(hepatico)jejunostomy or choledochoduodenostomy (Watanapa & Williamson, 1992). In the past, cholecystojejunostomy was preferred because it is a relatively simple procedure (Rappaport & Villalba, 1990). In an extensive review, the success rate of cholecystojejunostomy to relieve obstructive jaundice was lower, however, compared with choledochojejunostomy (Watanapa & Williamson, 1992). A randomized controlled trial by Sarfeh and colleagues (1988) also showed that the technically more difficult choledochojejunostomy is preferred over a cholecystenterostomy, owing to the lower rate of recurrent jaundice and cholangitis and better patency of the bypass.
A choledochoduodenostomy is not recommended because it is generally believed that this drainage procedure frequently results in recurrent jaundice from local tumor ingrowth into the duodenum and the distal common bile duct, including the entrance of the cystic duct. A few cohort studies show low procedure-related complication rates and low rates of recurrent jaundice after choledochoduodenostomy (Di Fronzo et al, 1999).
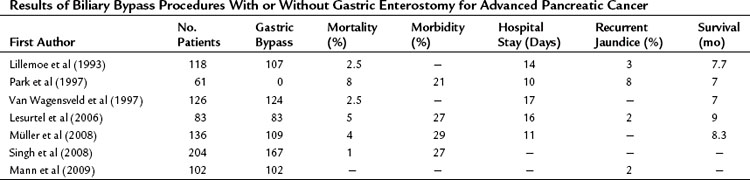
At our institution, a side-to-side Roux-en-Y choledochojejunostomy is routinely performed after removal of the gallbladder in case of detection of advanced disease or metastases. According to the extension of dissection, in an attempt to prove locally advanced disease by ingrowth at the proximal portal vein, the common bile duct may be transected in an early phase of the procedure, and an end-to-side anastomosis is made by a one-layer running suture. This procedure can be performed with a low mortality rate and acceptable morbidity. The mortality rate in earlier studies, as well as most “outdated” randomized controlled trials from the 1990s (see below), was high and varied between 15% and 24%. However, recent studies showed mortality rates between 1% and 8%, morbidity rates between 21% and 29%, and shorter hospital stay (10 to 17 days) with a relatively low incidence of recurrent jaundice. The mean survival varied between 7 and 9 months (Table 63B.1).
Surgical or Endoscopic or Percutaneous Drainage of Obstructive Jaundice
Five prospective, randomized controlled trials have been performed, four of which compared surgical biliary drainage and endoscopic drainage (Andersen et al, 1989; Bornmann et al, 1986; Nieveen van Dijkum et al, 2003; Shepherd et al, 1988; Smith et al, 1994). In the first trial by Bornmann and associates (1986), percutaneous biliary drainage was used, and no differences were found between percutaneous and surgical palliation (Table 63B.2). The other studies were performed between 1988 and 1994, except for the study done by Nieveen van Dijkum and colleagues (2003), in which patients underwent a diagnostic laparoscopy as a final staging procedure, and randomization was performed after pathology had proven metastasis. The small number of patients randomized hampered the studies by Shepherd and associates (1988) and Andersen and colleagues (1989). Shepherd and colleagues predominantly used 7-Fr endoprotheses, which are known to have a higher occlusion rate than 10- or 12-Fr endoprotheses or Wallstents currently used (Davids et al, 1992). A study by Shepherd and colleagues (1988) had a relatively high rate of recurrent jaundice and cholangitis. The length of follow-up in both studies was unclear, and the registration of complications and the readmission rate were limited. In the study by Smith and coworkers (1994), 201 patients were randomized. A higher procedure-related mortality rate was found after bypass compared with stenting (14% vs. 3%), and the 30-day mortality rate was not significantly different (8% vs. 15%) but was still relatively high. Major complications after bypass versus stenting were significantly different (29% vs. 11%), but minor complications were comparable (29% vs. 18%). The recurrence of jaundice and cholangitis during follow-up was significantly higher after stenting (36% vs. 2%), but survival was comparable between groups (Smith et al, 1994).
Table 63B.2 Prospective Randomized, Controlled Trials Comparing Percutaneous or Endoscopic Drainage with Surgical Biliary Drainage
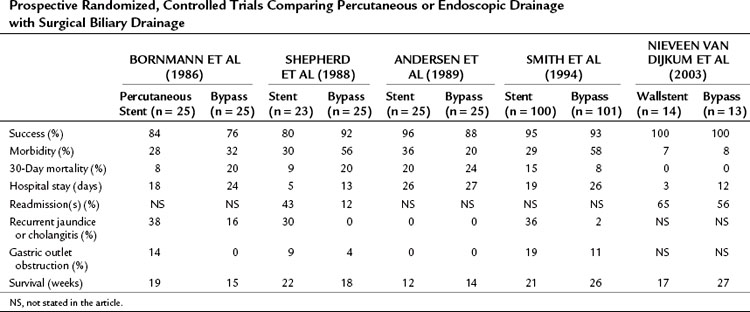
Taylor and associates (2000) conducted a meta-analysis using the three above-mentioned studies of endoscopic stenting and concluded that more treatment sessions were required after stent placement than after surgery, with a common odds ratio (OR) estimated to be 7.23. This ratio indicates a more than sevenfold increased risk of additional treatment sessions after stenting. No significant difference between the two treatment strategies was found concerning 30-day mortality rate.
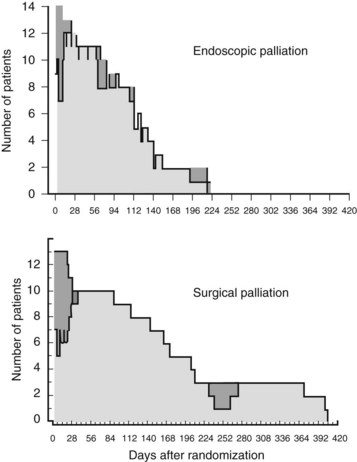
The more recent randomized study by Nieveen van Dijkum and colleagues (2003) analyzed the value of diagnostic laparoscopy for patients with a periampullary carcinoma. Patients who were found to have incurable disease owing to pathology-proven metastases were allocated to either surgical (double bypass) or endoscopic palliation by a Wallstent. No difference was found in procedure-related morbidity or number of readmitted patients between the surgically and endoscopically palliated patients (see Table 63B.2). The survival was 192 days and 116 days in the surgical and endoscopic groups, respectively (P = .05; Fig. 63B.2). This study concerns a select group of patients who were believed to have a resectable tumor after conventional radiologic staging, leading to a low (zero) procedure-related mortality.
Gastric Outlet Obstruction
Symptoms of GOO, such as nausea and vomiting, are reported in 11% to 50% of patients with pancreatic cancer at the time of diagnosis (DiMagno et al, 1999). For the optimal palliative treatment, it is important to determine the origin of these symptoms. The first cause is motility dysfunction of the stomach and duodenum secondary to tumor infiltration of the celiac nerve plexus (Thor et al, 2002) or probably dysfunction of the small bowel owing to tumor infiltration around the mesenteric artery. The second cause of GOO is mechanical obstruction of the duodenum from direct tumor ingrowth into the duodenum or secondary to compression of the duodenum by a tumor in the direct vicinity. At presentation, mechanical obstruction is reported in only approximately 5% of patients with pancreatic or periampullary tumors.
Surgical palliation should be performed only when GOO has a mechanical cause. When there is no mechanical cause, pharmaceutical treatment options should be investigated (Yeo et al, 1993). It is important to confirm mechanical GOO radiologically or endoscopically. Approximately 3% to 20% of patients with unresectable pancreatic cancer eventually develop mechanical GOO (Espat et al, 1999; Lillemoe et al, 1999). In a review by Watanapa and Williamson (1992) that included 1086 patients, 17% developed mechanical GOO, and an additional gastrojejunostomy was performed on these patients. In patients who are found to have an unresectable tumor at laparotomy, a gastrojejunostomy, in addition to a biliary bypass, is often performed and is not associated with increased morbidity and mortality (see Table 63B.1).
Endoscopic duodenal stenting also is accepted as a nonsurgical palliative treatment of duodenal obstruction (Holt et al, 2004; Telford et al, 2004). In a multicenter study, the success rate after stent placement was 84%, and oral intake in patients with successful stent placement resumed for a median time of 146 days (Telford et al, 2004). Recently, a systematic review was performed to analyze medical outcome and costs of gastrojejunostomy and stent placement. No difference was found in technical success rate (96% vs. 100%), early and late major complications (7% vs. 6% and 18% vs. 17%, respectively), and persisting symptoms (8% vs. 9%). Hospital stay was longer after surgical gastrojejunostomy, 13 versus 7 days after stent placement. The mean survival time after gastrojejunostomy was 164 days versus 105 days after stenting. The authors concluded that a stent should be used for patients with a relatively short life expectancy, and gastrojejunostomy should be performed for patients with a prolonged prognosis (Jeurnink et al, 2007).
Among surgeons and gastroenterologists, it is debated whether a prophylactic gastrojejunostomy should be performed. This is an important topic because only 40% to 80% of patients with pancreatic cancer who initially undergo an exploratory laparotomy with the intention to perform a pancreatoduodenectomy undergo a resection (Alexakis et al, 2004; Kuhlmann et al, 2004; Pisters et al, 2001; Sohn et al, 1999). Two randomized controlled trials evaluated the role of a prophylactic gastrojejunostomy in patients who were found to have an unresectable periampullary or pancreatic tumor during exploratory laparotomy (Lillemoe et al, 1999; Van Heek et al, 2003). Lillemoe and colleagues (1999)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree