Chapter 51 Palliation of Malignant Pancreaticobiliary Obstruction
![]() Video related to this chapter’s topics: Palliation of Malignant Biliary Obstruction with Self-Expanding Metallic Stent
Video related to this chapter’s topics: Palliation of Malignant Biliary Obstruction with Self-Expanding Metallic Stent
Epidemiology
Of all pancreaticobiliary malignancies, pancreatic adenocarcinoma has the highest incidence, with around 30,000 new cases annually in the United States. It ranks fifth among the leading causes of cancer-related deaths.1,2 Only 10% of patients are suitable candidates for resection, and the overall 5-year survival rate is less than 4%.3,4 The incidence of gallbladder carcinomas is 1 per 100,000 person-years. The survival rate is only slightly higher than that of pancreatic carcinoma.2 Patients most likely to survive are patients in whom early cancer was detected in a postcholecystectomy specimen. Klatskin’s tumors also have a poor prognosis, with less than 10% of patients surviving 5 years after being diagnosed and most patients dying in the first year.5 The number of potentially resectable tumors is low, ranging from 10% to 20%. In ampullary carcinoma, biliary obstruction usually develops early in the course of the disease. Tumors are usually small, and radical resection is possible in most cases, with an overall 5-year survival rate of 50%.6
Pathogenesis
A detailed discussion of the pathogenesis of pancreaticobiliary malignancies is beyond the scope of this chapter; however, several epidemiologic studies have identified risk factors for the development of pancreaticobiliary malignancies. Tobacco smoking doubles the risk of pancreatic cancer.7,8 Patients with chronic pancreatitis have an increased risk for developing pancreatic cancer that is estimated at 4% per 20 years.9 The risk of developing pancreatic cancer in patients with hereditary pancreatitis is 50%, with smoking as an important risk modifier.10,11 Etiologic factors for cholangiocarcinoma include primary sclerosing cholangitis and hepatolithiasis.12,13 Gallstone disease is the most important risk factor for gallbladder cancer.14
Pathology
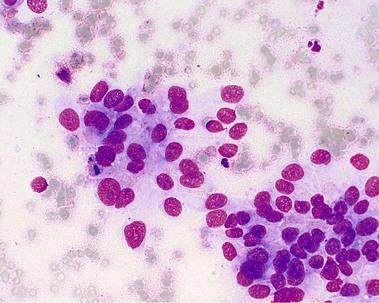
About 90% of pancreaticobiliary malignancies are ductal adenocarcinomas (Fig. 51.1). Most of these tumors arise from the pancreatic head. Other exocrine malignancies are mucinous cyst adenocarcinoma and acinar cell carcinomas. Endocrine tumors include gastrinoma and insulinoma. Metastases of a primary tumor (mammary, lung, and melanoma) and lymphoma should be considered because of important treatment implications (e.g., chemotherapy). Mesenchymal tumors are extremely rare.
Cytologic brushings are easy to obtain and widely used. Specificity approaches 100%, but sensitivity is 30% to 60%.15,16 The sensitivity in cholangiocarcinoma is greater than in pancreatic carcinoma. Forceps biopsy requires endoscopic sphincterotomy and is associated with a slightly increased risk of complications.17 Biopsy specimens of ampullary tumors can be obtained directly. Sampling of ductal fluid is a simple method, but the sensitivity is very low, and it is not used very often. Several studies have shown that sensitivity can be increased by combining different techniques of tissue sampling.16,18 Endoscopic ultrasound (EUS)–guided fine needle aspiration biopsy has an excellent sensitivity of 85% to 90% and specificity of virtually 100%.19 Although these tests may be useful in making the diagnosis of carcinoma, a negative test cannot rule out malignant disease. Percutaneous fine needle aspiration biopsy is another accurate method for confirmation of malignancy, with a sensitivity of 60% to 90%.20 However, needle tract seeding has been described, and this technique should be used only for tissue confirmation in the case of unresectable disease.
Differential Diagnosis
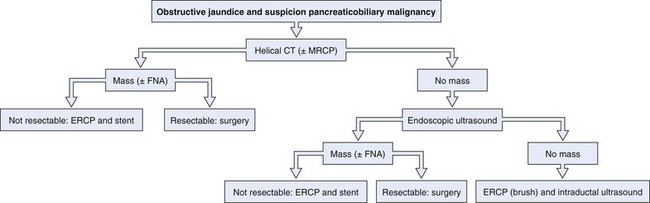
An enlarged pancreatic head may be caused either by pancreatitis or by carcinoma. The patient’s history and clinical presentation contribute to making a diagnosis. Autoimmune pancreatitis is an increasingly recognized condition and may mimic a malignant tumor. Differential diagnosis is based on a distinct clinical picture with a diffusely enlarged pancreas with a nondilated pancreatic duct, elevated IgG4 levels, and a prompt response to corticosteroid therapy with improvement of clinical symptoms including jaundice and resolution of morphologic abnormalities on cross-sectional imaging.21 Cystic lesions of the pancreas may be benign (pancreatic pseudocyst or serous cystadenoma), premalignant (mucinous cystadenoma), or malignant (cystadenocarcinoma). Radiologic imaging is used to characterize these lesions. EUS in combination with fine needle aspiration and fluid analysis may increase accuracy of the diagnosis further. In the case of a suspicious stricture in the mid–bile duct or proximal bile duct, a gallbladder carcinoma should be included in the differential diagnosis. It is important to exclude benign causes of strictures, such as Mirizzi’s syndrome, primary and secondary sclerosing cholangitis, and postoperative conditions. An algorithm for the diagnosis of pancreaticobiliary cancer is presented in Fig. 51.2.
Treatment
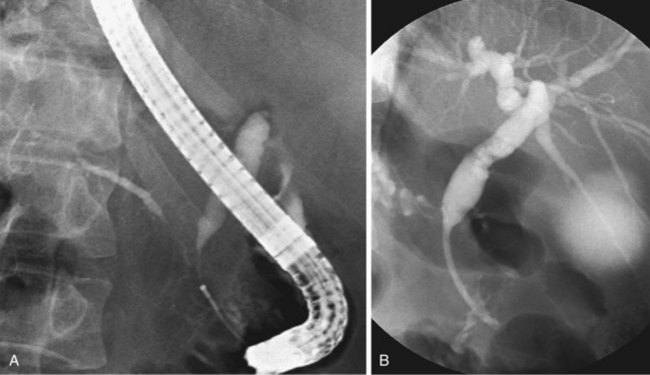
Since the introduction of endoscopic biliary stent therapy in 1980, the palliative treatment of pancreaticobiliary malignancies has changed considerably. At the present time, endoscopic stent placement to relieve jaundice is well established and is considered the preferred treatment (Fig. 51.3). Compared with percutaneous and surgical drainage, endoscopic biliary stent therapy is associated with lower morbidity and mortality rates.22–24 The main problem of endoscopic biliary drainage is late stent occlusion, which necessitates stent exchange. The technical success rate of endoscopic biliary drainage is 70% to 90% and is higher for distal tumors compared with more proximal malignancies involving the bifurcation. The complication rate of therapeutic ERCP is 5% to 10%.25,26
Indications and Contraindications
The indications for ERCP with a drainage procedure by stent placement include jaundice, fever, and pruritus. Biliary stent placement has also been shown to improve symptoms of anorexia and quality of life.27,28 It has been suggested that preoperative biliary drainage may improve surgical outcome after pancreaticoduodenectomy, but this has not been substantiated in clinical trials.29,31 In a randomized study comprising 202 patients with pancreatic head carcinoma comparing direct surgery with delayed surgery after biliary drainage, surgical outcome and complication rates were not affected by preoperative stent placement. The overall complication rate in the delayed surgery group with preoperative stent placement was significantly increased, mainly owing to stent-related complications. The outcome of this study strongly argues against standard preoperative drainage in patients with pancreatic head cancer in whom immediate surgery is planned. Preoperative drainage is indicated, however, when operative resection is not imminent. One common example is preoperative drainage performed with neoadjuvant chemotherapy. Some authors argue for preferential insertion of an expandable metal stent owing to their comparable durable patency.32,33 There are no absolute contraindications. Coagulation disorders are a relative contraindication and should be corrected before ERCP.
Overview of Stents for Biliary Drainage
Plastic Stents
The median patency of a conventional 10-Fr plastic stent is 3 to 6 months. The incidence of stent occlusion is 20% to 50%.34–36 The initial event in stent blockage is adherence of proteins and bacteria to the inner wall of the stent and subsequent formation of a biofilm. Bacteria are introduced into the biliary system during transpapillary placement of the stent. Sludge forms from the accumulation of bacteria, which produce β-glucuronidase and form calcium bilirubinate and calcium palmitate.37–39 Many efforts have been made to prolong stent patency, some of which are discussed in the following paragraphs.
Stent Diameter
The first biliary stents that were placed were only 7-Fr or 8-Fr in diameter because of limitations of the diameter of the working channel of the endoscope (2.8 mm). When side-viewing endoscopes with large-diameter working channels (4.2 mm) were introduced in 1980, it became possible to insert large-bore plastic stents.40 Larger stents (10-Fr) perform better than smaller stents (7-Fr)41 apparently because of the higher flow rate, as predicted by Poiseuille’s law, and less stasis with larger diameter stents. Theoretically, bile flow rate is proportional to the internal diameter raised to the fourth power; even a small increase in diameter results in a substantial increase in flow capacity.42 In contrast to this hypothesis, the use of larger diameter plastic stents of 11.5 Fr or 12 Fr did not result in further improvements in stent patency.43–45
Stent Design
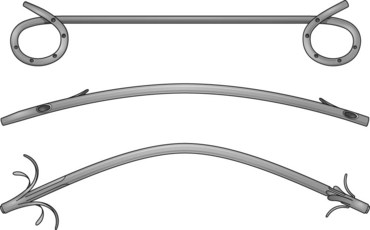
The first biliary stents had a pigtail configuration at the proximal end to provide better anchorage. Straight stents were developed because of their improved bile flow characteristics compared with pigtail stents (Fig. 51.4).42,46,47 Huibregtse and Tytgat48 developed the Amsterdam-type stent—a straight design with two side holes to facilitate biliary drainage and two side flaps to prevent dislocation—which has been the standard type of stent since 1980.
Sludge in plastic stents mainly accumulates around side holes.37,49 This accumulation seems to be the result of higher intraluminal flow turbulence and decreased flow rates.42 Soehendra and others50,51 postulated that elimination of side holes might improve patency rates and designed the so-called Teflon Tannenbaum stent (a straight stent without side holes and with multiple proximal and distal side flaps to prevent dislocation). At first, uncontrolled results were encouraging, with patency rates comparable to metal stents, but randomized trials could not confirm these initial results.52–54 Omitting side holes in a standard-design polyethylene stent also did not improve stent patency.55
Stent Material
Different materials have been used for stent construction, including polyethylene, polyurethane, and polytef (Teflon). In vitro studies have shown a direct relationship between the coefficient of friction and the amount of encrusted material. Teflon has the lowest friction coefficient and the best potential for preventing stent clogging.37 Initially, Teflon Tannenbaum stents showed a favorable patency rate.50,51 A randomized study comparing Amsterdam-type stents made from polyethylene and Teflon did not show a difference in stent patency.56 Other controlled clinical trials also could not confirm the superiority of Teflon material in a Tannenbaum-design stent.52–54
Scanning electron microscopy of out-of-package biliary stents has shown that the inner surface smoothness of plastic stents is highly variable. This variability is possibly a result of the manufacturing process of plastic stents by extrusion. Only the polyurethane stent was found to have an extremely smooth surface.57 Two new polymers were introduced with an ultrasmooth surface, Vivathane and Hydromer. Both materials have been shown to reduce bacterial adherence in vitro.58,59 In addition, the Hydromer stent has not only a smooth texture but also a coating that absorbs water and provides a hydrophilic sheath. Because bacteria initially attach by hydrophobic interactions, this coating could potentially lower bacterial adhesion and increase stent patency. However, the encouraging results of in vitro studies could not be confirmed in prospective clinical trials.60,61
Stent Coating
Priming the inner surface of a stent with a coating comprising some form of antiadhesion property may reduce biofilm formation and stent clogging. Antibiotics, antithrombotics, silver, and hydrophilic coating all were effective in reducing bacterial colonization in vitro.59,62,63 However, clinical studies using antibiotic-coated or hydrophilic-coated stents did not show any benefit.61
Stent Position
Placing the stent entirely within the common bile duct has the theoretical advantage of preserving the barrier function of the sphincter of Oddi; this prevents duodenal reflux of food and bacteria into the stent and biliary tree. This so-called inside stent approach can be performed only when a free margin of 1 to 2 cm is maintained between the distal end of the stricture and the papilla. With this parameter in mind, about one-third of patients with malignant obstructive jaundice are potential candidates for such treatment.64 However, no difference was found in stent performance in a randomized trial. In the inside stent group, stent migration occurred significantly more often.65
Antibiotics
In vitro studies showed that antibiotic treatment reduced bacterial adherence to plastic stents.66 In a prospective randomized study with ciprofloxacin, no difference in stent patency was found.67 In another study, rotating antibiotics (cycles of 2 weeks of ampicillin, metronidazole, and ciprofloxacin) were combined with ursodeoxycholic acid, and no difference in stent patency was shown.68 Only one small pilot study showed a reduced rate of stent blockage with norfloxacin plus ursodeoxycholic acid.69 Other studies combining antibiotics and bile salts (ofloxacin and ursodeoxycholic acid, ciprofloxacin, and Rowachol) did not show a longer duration of stent patency.70,71 There is no compelling evidence that stent patency benefits from antibiotic prophylaxis.
Aspirin
Animal studies in prairie dogs showed that aspirin inhibits mucous glycoprotein secretion by blocking prostaglandin synthesis.72 In a clinical study, the use of aspirin reduced the content of all sludge components, although no effect was shown on stent patency.73 No further studies using aspirin have been performed.
Bile Salts
Bile salts have a potent antibacterial effect and may stimulate bile flow. Because bacteria attach by hydrophobic interactions, hydrophobic bile salts (deoxycholate, taurodeoxycholate) inhibit initial bacterial attachment, as was shown in experimental studies.74 However, hydrophobic bile salts are not well tolerated. Hydrophilic bile salts such as ursodeoxycholate, which are better tolerated, have a minimal effect on bacterial adhesion. Except for one small pilot study, different prospective clinical studies using ursodeoxycholic acid alone or combining ursodeoxycholic acid with antibiotics could not show a difference in stent patency.68–71
Stent Exchange
Some endoscopists prefer to schedule patients for elective stent exchange every 3 to 4 months. The optimal time interval is unknown.75,76 Prophylactic stent exchange requires a repeat (clinically not indicated) endoscopy and has to be compared with the risks of watchful waiting and the risk of (severe) cholangitis. Because most patients do not develop stent occlusion before dying of the underlying disease, most endoscopists favor an expectant management strategy.
Stent Cleaning
Some endoscopists have proposed leaving an occluded stent in situ and cleaning the obstructed lumen with a cytology brush or flushing with saline instead of performing stent replacement.77 However, stent cleaning carries the risk of inducing biliary sepsis by actively introducing the biofilm of the stent and bacteria from the duodenum into the biliary tract. Stent cleaning is not recommended.
Self-Expanding Metal Stent
The diameter of biliary stents was restricted by the size of the instrumentation channel of the endoscope until the development of self-expanding metal stents. All currently available expandable stents are made of metal. They differ in the way they are braided, the size of the mesh, the metal used, and their rigidity. At the present time, different types of self-expanding metal stents are available from various manufacturers (Fig. 51.5). To date, the most experience has been gained with the self-expanding Wallstent (Boston Scientific, Natick, MA). This stent is delivered in a collapsed configuration on an 8-Fr delivery system. When deployed, it expands to a final diameter of 30 Fr (approximately 10 mm) and shortens about 30% in length. The final diameter is achieved after 1 week, when equilibrium is achieved between the dilating force of the stent and the resistance of the bile duct wall and tumor. These large-caliber self-expanding metal stents of 30 Fr remain patent for longer than plastic stents but do not prevent blockage indefinitely. Metal stents with a 6-mm diameter occlude significantly more frequently than 10-mm (30-Fr) metal stents, showing that size is the most important determining factor for stent patency.78
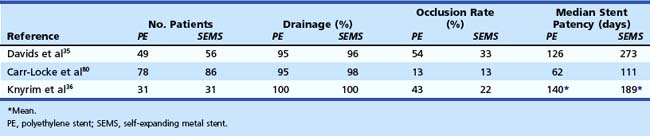
Because of their design, self-expanding metal stents have much less surface to which bacteria can adhere. The mechanism of stent blockage differs from that seen in plastic stents and includes tumor ingrowth through the interstices of the stent or overgrowth of the end of the stent and intima hyperplasia. Several studies have shown a median stent patency of about 6 to 9 months (Table 51.1).35,36,76,79,80 Self-expanding metal stents are more difficult to insert, uncovered metal stents cannot be removed after deployment, and initial costs are high (about $1000). Various types of self-expanding metal stents are available to date.
Wallstent
The initial endoscopic placement experience was reported in 1989.81 The Wallstent is made from stainless steel alloy filaments braided in a tubular mesh configuration. In the early phase of development, technical problems mainly involved the restraining membrane failing to retract completely, but this is now rarely seen.82 The first randomized trial comparing plastic stents and the Wallstent was performed by Davids and coworkers.35 Wallstent patency was superior to patency of plastic stents, with a median duration of 9 months. These results were confirmed in several other studies.36,76,83 The Wallstent is offered in two diameters (8 mm and 10 mm) and various lengths (40 mm, 60 mm, 80 mm, and 100 mm). The Wallstent is also available with a covering designed to resist tumor ingrowth.